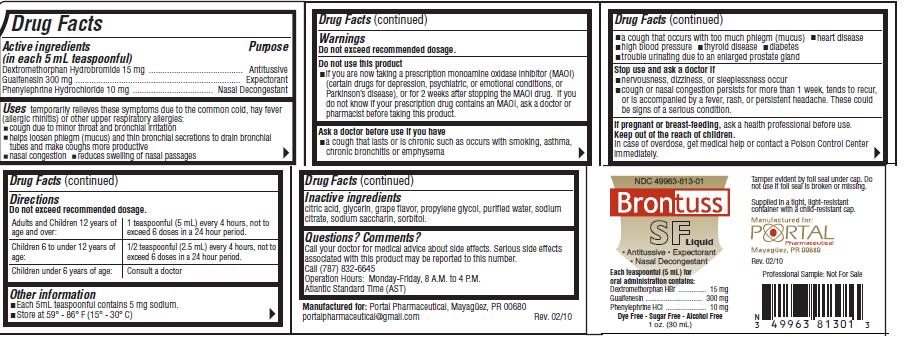
Drug Facts
Active ingredients Purpose
(in each 5 mL teaspoonful)
Dextromethorphan Hydrobromide 15 mg..................Antitussive
Guaifenesin 300 mg...............................................Expectorant
Phenylephrine Hydrochloride 10 mg.........................Nasal Decongestant
Uses
temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:- cough due to minor throat and bronchial irritation
- helps losen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- nasal congestion
- reduces swelling of nasal passages
Warnings
Do not exceed recommended dosage.Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Directions
Do not exceed recommended dosage.| Adults and Children 12 years of age and over: | 1 teaspoonful (5 mL) every 4 hours, not to exceed 6 doses in a 24 hour period. |
| Children 6 to under 12 years of age: | 1/2 teaspoonful (2.5 mL) every 4 hours, not to exceed 6 doses in a 24 hour period. |
| Children under 6 years of age: | Consult a doctor |
Inactive Ingredients
citric acid, glycerin, grape flavor, propylene glycol, purified water, sodium citrate, sodium saccharin, sorbitol.Questions? Comments?
Call your doctor for medical advise about side effects. Serious side effects associated with this productmay be reported to this number.
Call (787) 832-6645
Operation Hours: Monday-Friday, 8 A.M. to 4 P.M.
Atlantic Standard Time (AST)
Manufactured for: Portal Pharmaceuticals, Mayaguez, PR 00680
portalpharmaceutical@gmail.com Rev. 02/10
Product Packaging:
Packaging below represents the labeling currently used:Principal display panel and side panel for 30 mL label:
NDC 49963-813-01
Brontuss SF Liquid
Antitussive/Expectorant/Nasal Decongestant
Each teaspoonful (5 mL) for
oral administration contains:
Dextromethorphan HBr.........................15 mg
Guaifenesin.......................................300 mg
Phenylephrine HCl...............................10 mg
Dye Free/Sugar Free/Alcohol Free
1 oz. (30 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Supplied in a tight, light-resistant container with a child-resistant cap.
Manufactured for:
PORTAL Pharmaceutical
Mayaguez, PR 00680 Rev. 02/10
Principal display panel and side panel for 118 mL label:
NDC 49963-813-04
Brontuss SF Liquid
Antitussive/Expectorant/Nasal Decongestant
Each teaspoonful (5 mL) for
oral administration contains:
Dextromethorphan HBr..........................15 mg
Guaifenesin........................................300 mg
Phenylephrine HCl................................10 mg
Dye Free/Sugar Free/Alcohol Free
4 oz. (118 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Supplied in a tight, light-resistant container with a child resistant cap.
Manufactured for:
PORTAL Pharmaceutical
Mayaguez, PR 00680
Rev. 02/10