Warnings
For external use only
Do not use
- more than one patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any igredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Directions
Adults and children 12 years of age and over:
- clean and dry affected area
- remove film from patch and apply to the skin (see illustration)
- apply to affected are not more than 3 to 4 times daily
- remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
Inactive ingredients
aluminum silicate, dihydroxyaluminum aminoacetate, disodium edetate, gelatin, glycerin, methylparaben, oleic acid, polyacrylic acid, polyvinyl alcohol, propylene glycol, propylparaben, sodium polyacrylate, tartaric acid, titanium dioxide, water
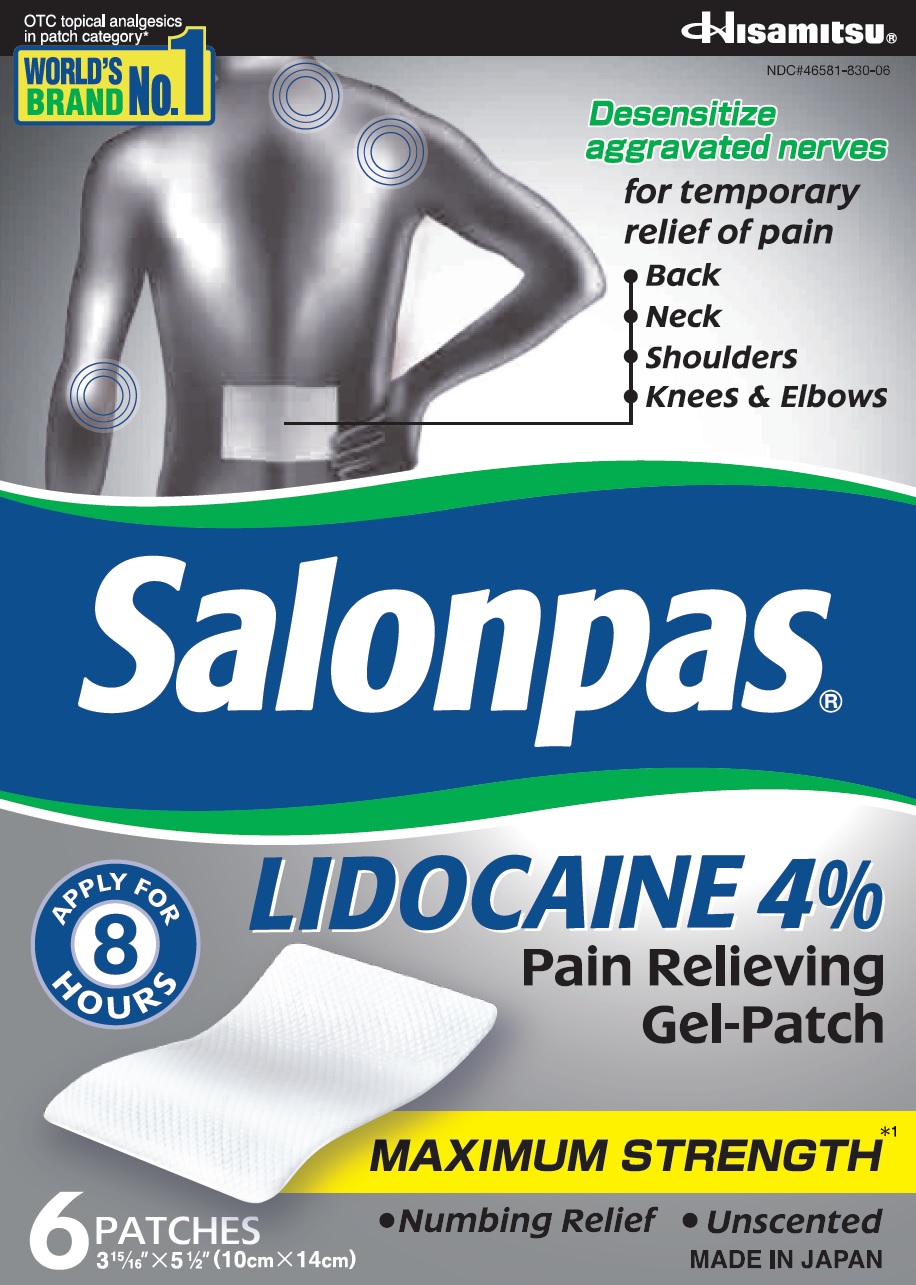
Principal Display Panel
OTC topical analgesics in patch category*
WORLD'S BRAND NO.1
Hisamitsu
NDC#46581-830-06
Desensitize aggravated nerves
for temporary relief of pain
- Back
- Neck
- Shoulders
- Knees and Elbows
Salonpas LIDOCAINE 4% Pain Relieving Gel-Patch
APPLY FOR 8 HOURS
MAXIMUM STRENGTH*1
- Numbing Relief
- Unscented
6 PATCHES
3 15/16" X 5 1/2" (10cm X 14cm)
MADE IN JAPAN
Principal Display Panel
CUT OPEN HERE
NDC#46581-830-01
HOW TO APPLY
1 Pull apart.
2 Peel off one side of the film.
3 Place on affected area.
4 Peel off the remaining film.
5 Press patch thoroughly.
Salonpas LIDOCAINE 4% Pain Relieving Gel-Patch
APPLY FOR 8 HOURS
MAXIMUM STRENGTH*1
- Numbing Relief
- Unscented
1 PATCH
3 15/16" X 5 1/2" (10cm X 14cm)
Child-resistant packaging.
Hisamitsu
MADE IN JAPAN
