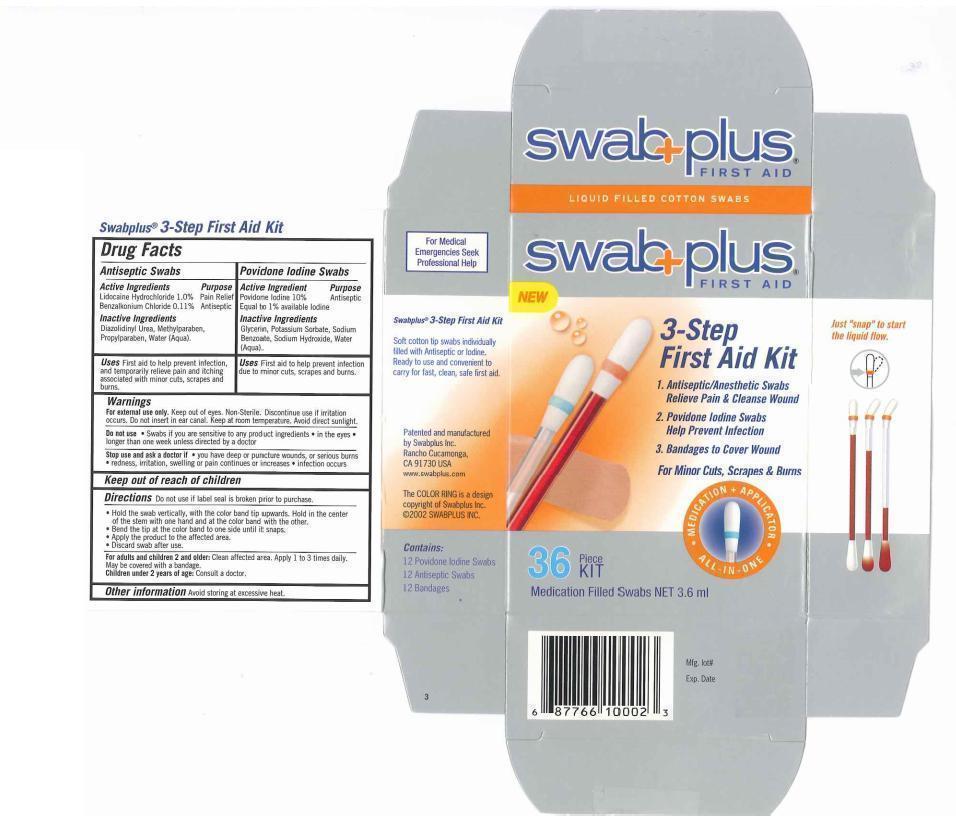
Drug Facts
Active Ingredients
Antiseptic Swabs
Lidocaine Hydrochloride 1.0%
Benzalkonium Chloride 0.11%
Povidone Iodine swabs:
Povidone Iodine 10%, equal to available Iodine 1% for antiseptic
Purpose
Lidocaine Hydrochloride for Pain Relief
Benzalkonium Chloride for Antiseptic
Povidone Iodine for Antiseptis
Uses
First aid to help prevent infection, and temporarily reliever pain and itching associated with minor cuts. scrapes and burn
Directions
- Do not use if label seal is broken prior to purchase.
- Hold the swab vertically, with the color ring band tip upwards. Hold in the center of the stem with one hand and at the color band with the other.
- Bend the tip at the color band to one side until it snaps.
- Apply the product to the affected area.
- Discard swab after use.
Warnings
For external use only. Keep out of eyes. Non-Sterile. Discontinue use if irritation occurs. Do not insert inear canal. Keep at room temperture. Avoid direct sunlight.
Do not use. Swabs if you are sensitive to any product ingredients. in the eyes. longer than one week unless directed by a doctor.
Stop use and ask a doctor if you have deep or punchture wounds, or serious burns. redness, irritation, sweeling or pain continues or increases, infection occurs
Other information
For adult and children 2 and older: Clean affected area. Apply 1 to 3 times daily. May be covered with a bandage.
Children under 2 years of age: Consult a doctor.
Avoid storing at excessive heat.
Inactive Ingredients
Antiseptic swab: Dizolidinyl Urea, Methylparaben, Propylparaben, Water
Providone Iodine Swab: Glcerin, Water
Manufacturer Statement
Soft cotton tip swabs individually filled with Antiseptic or Iodine. Ready to use and convenient to carry for fast, clean, safe first aid.
Patented and manufactured by Swabplus Inc. Rancho Cucamonga, CA 91730 USA
The COLOR RING is copyrighted by Swabplus Inc. ©2007 SWABPLUS INC.