HBS Code (Labeler): FP40901-30.2
Distributor: CVS Pharmacy, Inc.
Human OTC Drug
Active Ingredients: Salicylic Acid 2%
CARTON TEXT
________________________________________________________
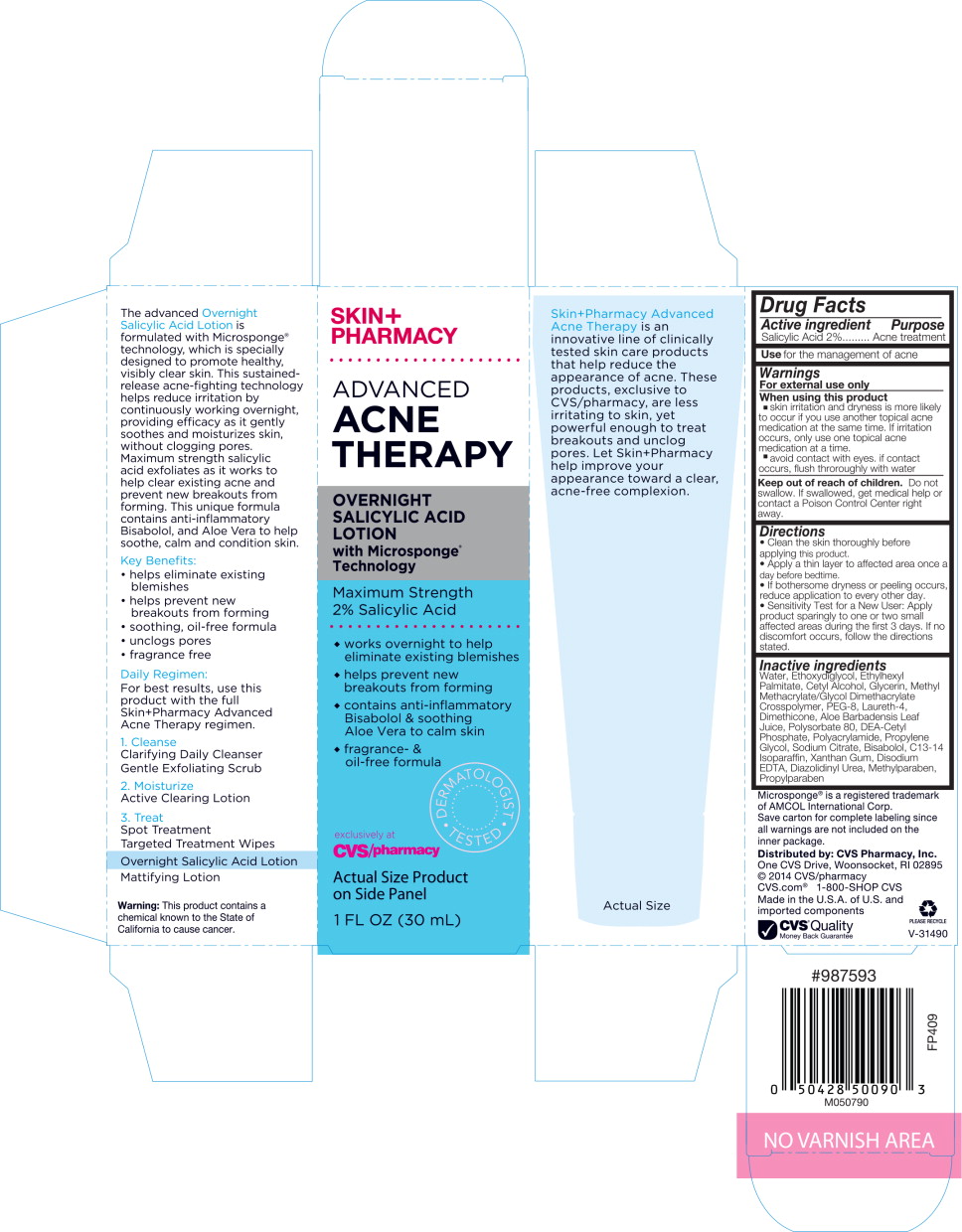
SKIN+PHARMACY
ADVANCED ACNE THERAPY
OVERNIGHT SALICYLIC ACID LOTION with Microsponge® Technology
Maximum Strength 2% Salicylic Acid
- works overnight to help eliminate existing blemishes
- helps prevent new breakouts from forming
- contains anti-inflammatory Bisabolol & soothing Ale Vera to calm skin
- fragrance- & oil-free formula
DERMATOLOGIST TESTED
exclusively at CVS/pharmacy
Actual Size Product on Side Panel
1 FL OZ (30 mL)
The advanced Overnight Salicylic Acid Lotion is formulated with Microsponge® technology, which is specially designed to promote healthy, visibly clear skin. This sustained-release acne-fighting technology helps reduce irritation by continuously working overnight, providing efficacy as it gently soothes and moisturizes skin, without clogging pores. Maximum strength salicylic acid exfoliates as it works to help clear existing acne and prevent new breakouts from forming. This unique formula contains anti-inflammatory Bisabolol, and Aloe Vera to help soothe, calm and condition skin.
Key Benefits:
- helps eliminate existing blemishes
- helps prevent new breakouts from forming
- soothing, oil-free formula
- unclogs pores
- fragrance free
Daily Regiment:
For best results, use this product with the full Skin+Pharmacy Advanced Acne Therapy regimen.
- Cleanse
Clarifying Daily Cleanser Gentle Exfoliating Scrub - Moisturize
Active Clearing Lotion - Treat
Spot Treatment
Targeted Treatment Wipes
Overnight Salicylic Acid Lotion
Mattifying Lotion
Warning: This product contains a chemical known to the State of California to cause cancer.
Skin+Pharmacy Advanced Acne Therapy is an innovative line of clinically tested skin care products that help reduce the appearance of acne. These products, exclusive to CVS/pharmacy, are less irritating to skin, yet powerful enough to treat breakouts and unclog pores. Let Skin+Pharmacy help improve your appearance toward a clear, acne-free complexion.
Actual Size
Drug Facts
Warnings
For external use only
Directions
- Clean the skin thoroughly before applying this product.
- Apply a thin layer to affected area once a day before bedtime.
- If bothersome dryness or peeling occurs, reduce application to every other day.
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated.
Inactive ingredients
Water, Ethoxydiglycol, Ethylhexyl Palmitate, Cetyl Alcohol, Glycerin, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, PEG-8, Laureth-4, Dimethicone, Aloe Barbadensis Leaf Juice, Polysorbate 80, DEA-Cetyl Phosphate, Polyacrylamide, Propylene Glycol, Sodium Citrate, Bisabolol, C13-14 Isoparaffin, Xanthan Gum, Disodium EDTA, Diazolidinyl Urea, Methylparaben, Propylparaben
Microsponge® is a registered trademark of AMCOL International Corp.
Save carton for complete labeling since all warnings are not included on the inner package.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and foreign components
V-13649
CVS® Quality
Money Back Guarantee
PLEASE RECYCLE
V-31490
#987593
FP409
M050790
________________________________________________________
end of text
160611
AMCOL Health & Beauty Solutions, Inc. DBA AMCOL Household and Personal Care
301 Laser Lane Lafayette, LA 70507 | www.amcolhpc.com
lauren.haase@mineralstech.com
Principal Display Panel - 20 g/2 mL Carton Label
SKIN +
PHARMACY
ADVANCED
ACNE
THERAPY
OVERNIGHT
SALICYLIC ACID
LOTION
with Microsponge*
Technology
Maximum Strength
2% Salicylic Acid
- works overnight to help
eliminate existing blemishes - helps prevent new
breakouts from forming - contains anti-inflammatory
Bisabolol & soothing
Aloe Vera to calm skin - fragrance- &
oil-free formula
exclusively at
CVS/pharmacy
Actual Size Product on Side Panel
1 FL OZ (30 mL)
Principal Display Panel - 20 g/2 mL Tube Label
SKIN +
PHARMACY
ADVANCED
ACNE
THERAPY
OVERNIGHT
SALICYLIC ACID LOTION
with Microsponge* Technology
Maximum Strength
2% Salicylic Acid
- works overnight to help eliminate
existing blemishes - helps prevent new breakouts
from forming - contains anti-inflammatory
Bisabolol & soothing Aloe Vera
to calm skin - fragrance- & oil-free formula
exclusively at
CVS/pharmacy
1 FL OZ (30 mL)