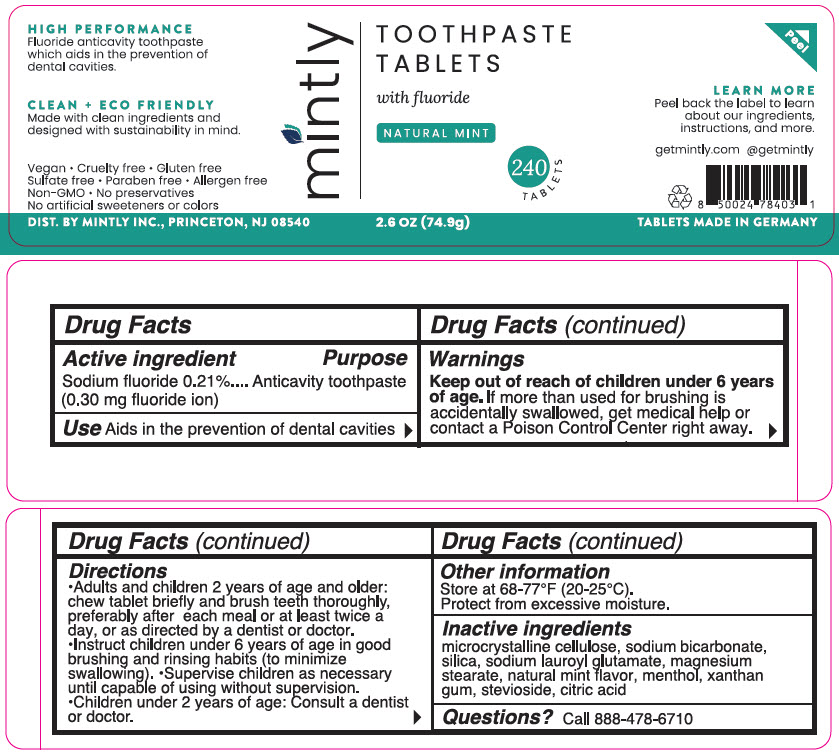
FLUORIDE- sodium fluoride tablet
Mintly Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Sodium fluoride 0.21% (0.30 mg fluoride ion)
Purpose
Anticavity toothpaste
Use
Aids in the prevention of dental cavities
Warnings
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older: chew tablet briefly and brush teeth thoroughly, preferably after each meal or at least twice a day or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or doctor.
Other information
Store at 68-77°F (20-25°C).
Protect from excessive moisture.
Inactive ingredients
microcrystalline cellulose, sodium bicarbonate, silica, sodium lauroyl glutamate, magnesium stearate, natural mint flavor, menthol, xanthan gum, stevioside, citric acid
Questions?
Call 888-478-6710
DIST. BY MINTLY INC., PRINCETON, NJ 08540
PRINCIPAL DISPLAY PANEL - 240 Tablet Jar Label
mintly
TOOTHPASTE
TABLETS
with fluoride
NATURAL MINT
240
TABLETS
2.6 OZ (74.9g)
Mintly Inc.