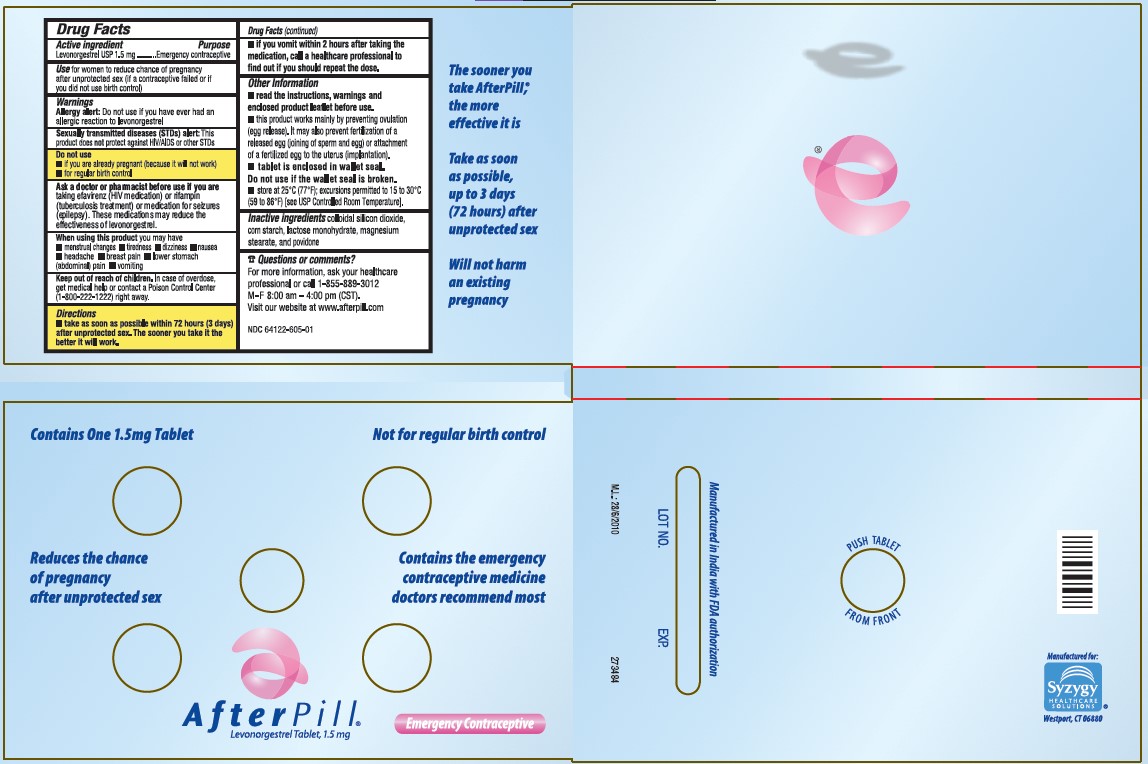
Indications
Use for women to reduce chance of pregnancy after unprotected sex (if a contraceptive failed or if you did not use birth control).
Warnings
Allergy alert: Do not use if you have ever had an allergic reaction to levonorgestrel.
Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other STDs
Do not use
- if you are already pregnant (because it will not work)
- for regular birth control
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
When using this product you may have
- menstrual changes
- nausea
- lower stomach (abdominal) pain
- tiredness
- headache
- dizziness
- breast pain
- vomiting
Keep out of the reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- take as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it the better it will work.
- if you vomit within 2 hours after taking the medication, call a healthcare professional to find out if you should repeat the dose.
- read the instructions, warnings and enclosed product leaflet before use .
- this product works mainly by preventing ovulation (egg release). It may also prevent fertilization of a released egg (joining of sperm and egg) or attachment of a fertilized egg to the uterus (implantation).
- tablet is enclosed in wallet seal. Do not use if the wallet seal is broken .
- store at 25°C (77°C); excursions permitted to 15 to 30°C (59 to 86°C) [see USP Controlled Room temperature].
Inactive Ingredients
colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, and povidone.
For more information, ask your healthcare professional or call 1-855-889-3012
M-F 8:00 am -4:00 pm (CST)
Visit our website at www.afterpill.com
(levonorgestrel) Tablet, 1.5 mg
Emergency Contraceptive
One Tablet. One Dose.
What You Need to Know
AfterPill® is emergency contraception that helps prevent pregnancy after birth control failure or unprotected sex. It is a backup method of preventing pregnancy and should not be used as regular birth control.
What AfterPill Solo is not.
AfterPill will not work if you are already pregnant and will not affect an existing pregnancy. AfterPill will not protect you from HIV infection (the virus that causes AIDS) and other sexually transmitted diseases (STDs).
When should I use AfterPill?
The sooner you take emergency contraception, the better it works. You should use AfterPill within 72 hours (3 days) after you have had unprotected sex.
AfterPill is a backup or emergency method of birth control you can use when:
- your regular birth control was used incorrectly or failed
- you did not use any birth control method
AfterPill should not be used:
- as a regular birth control method, because it's not as effective as regular birth control.
- if you are already pregnant, because it will not work.
- if you are allergic to levonorgestrel or any other ingredients in AfterPill.
When should I talk to a doctor or pharmacist?
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of AfterPill and increase your chance of becoming pregnant. Your doctor may prescribe another form of emergency contraception that may not be affected by these medications.
How does AfterPill work?
AfterPill works before release of an egg from the ovary. As a result, AfterPill usually stops or delays the release of an egg from the ovary. AfterPill is one tablet that contains a higher dose of levonorgestrel than birth control pills and works in a similar way to prevent pregnancy.
How can I get the best results from AfterPill?
You have 72 hours (3 days) to try to prevent pregnancy after birth control failure or unprotected sex. The sooner you take AfterPill, the better it works.
How effective is AfterPill?
If AfterPill is taken as directed, it can significantly decrease the chance that you will get pregnant. About 7 out of every 8 women who would have gotten pregnant will not become pregnant.
How will I know AfterPill worked?
You will know AfterPill has been effective when you get your next period, which should come at the expected time, or within a week of the expected time. If your period is delayed beyond 1 week, it is possible you may be pregnant. You should get a pregnancy test and follow up with your healthcare professional.
Will I experience any side effects?
- some women may have changes in their period, such as a period that is heavier or lighter or a period that is early or late. If your period is more than a week late, you may be pregnant.
- If you have severe abdominal pain, you may have an ectopic pregnancy, and should get immediate medical attention.
- when used as directed, AfterPill is safe and effective. Side effects may include changes in your period, nausea, lower stomach (abdominal) pain, tiredness, headache, dizziness, and breast tenderness.
- if you vomit within 2 hours of taking the medication, call a healthcare professional to find out if you should repeat the dose.
What if I still have questions about AfterPill?
If you have questions or need more information, call our toll-free number, 1-855-889-3012 or visit our website at www.afterpill.com.
Other Information
Keep this and all medication out of reach of children:
In case of overdose, get medical help or contact a Poison Control Center right away at 1-800-222-1222.
Do not use if the wallet seal is opened.
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature].
Active Ingredient: levonorgestrel 1.5 mg
Inactive Ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, and povidone.
AfterPill® is a registered trademark of Syzygy Healthcare Solutions, LLC.
Manufactured in India for
Syzygy Healthcare Solutions, LLC

If you are sexually active, you should see a healthcare provider for routine checkups. Your healthcare provider will talk to you about and, if necessary, test you for sexually transmitted diseases, teach you about effective methods of routine birth control, and answer any other questions you may have.