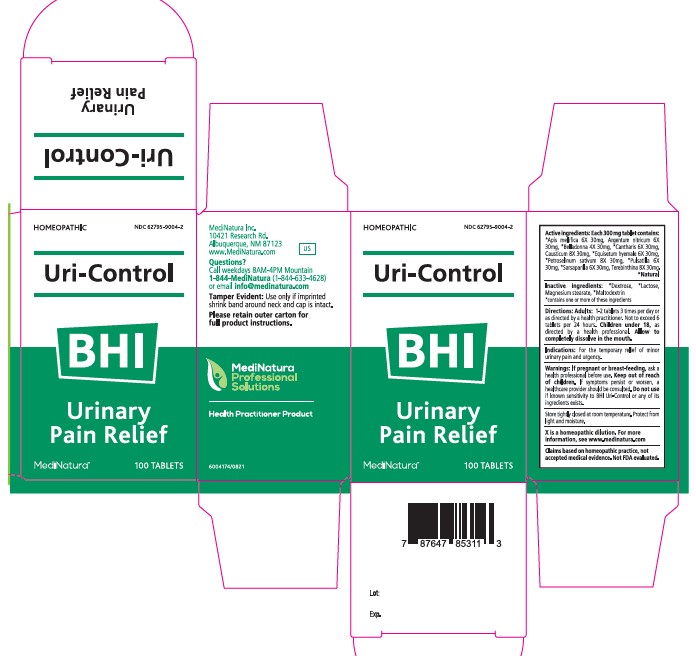
Warnings:
If pregnant or breast-feeding, ask a
health professional before use. Keep out of reach
of children. If symptoms persist or worsen, a
healthcare provider should be consulted. Do not use
if known sensitivity to BHI Uri-Control or any of its
ingredients exists.
Directions:
Adults: 1-2 tablets 3 times per day or
as directed by a health practitioner. Not to exceed 6
tablets per 24 hours. Children under 18, as
directed by a health professional. Allow to
completely dissolve in the mouth
Active ingredients:
Each 300 mg tablet contains:
*Apis mellifica 6X 30mg, Argentum nitricum 6X
30mg, *Belladonna 4X 30mg, *Cantharis 6X 30mg,
Causticum 8X 30mg, *Equisetum hyemale 6X 30mg,
*Petroselinum sativum 8X 30mg, *Pulsatilla 6X
30mg, *Sarsaparilla 6X 30mg, Terebinthina 8X 30mg.
*Natural