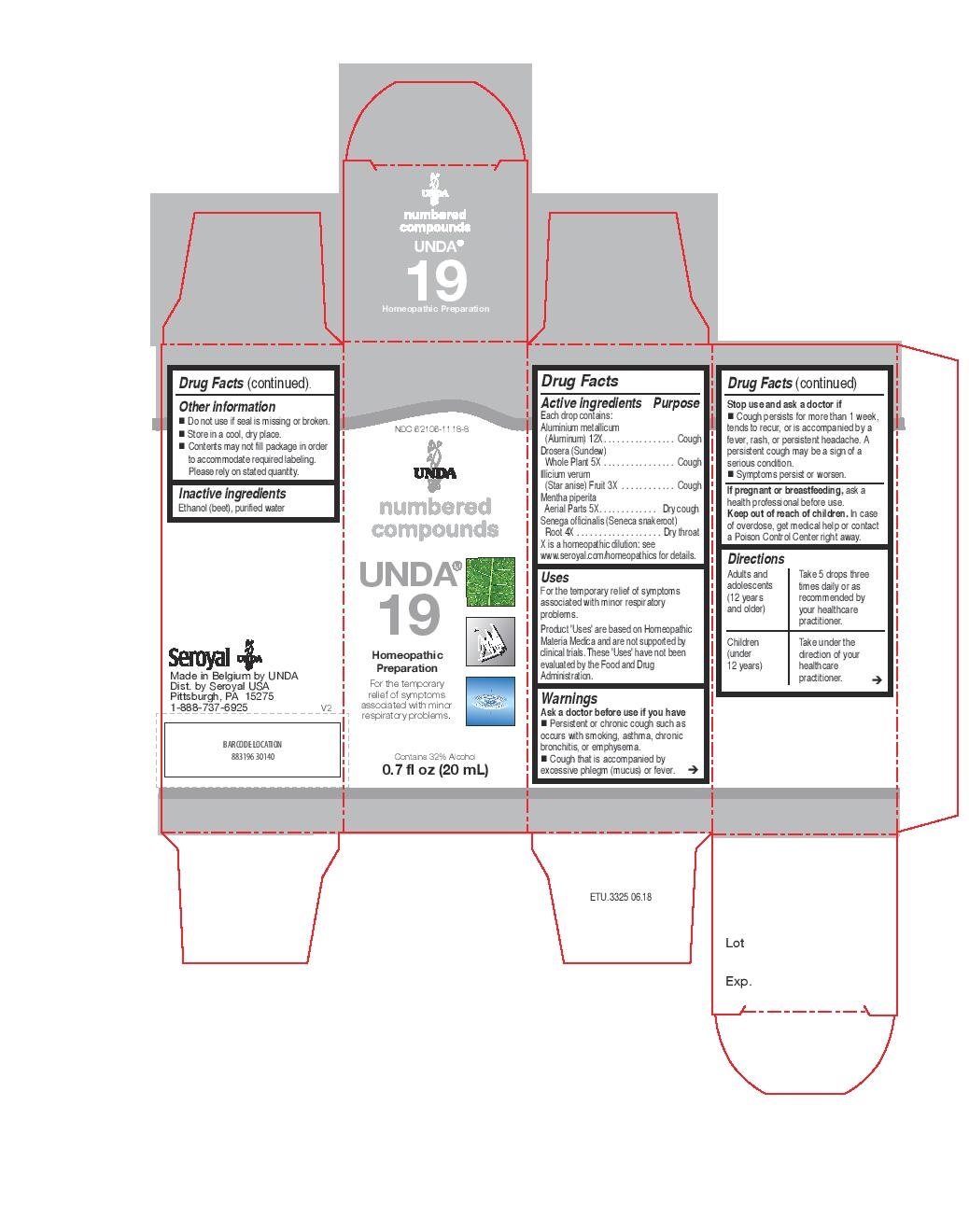
Active ingredients
Each drop contains:
Aluminium metallicum (Aluminum) 12X
Drosera (Sundew) Whole Plant 5X
Illicium verum (Star anise) Fruit 3X
Mentha piperita Aerial Parts 5X
Senega officinalis (Seneca snakeroot) Root 4X
Warnings
Ask a doctor before use if you have
Persistent or chronic cough such as
occurs with smoking, asthma, chronic
bronchitis, or emphysema.
Cough that is accompanied by
excessive phlegm (mucus) or fever.
Stop use and ask a doctor if
Cough persists for more than 1 week,
tends to recur, or is accompanied by a
fever, rash, or persistent headache. A
persistent cough may be a sign of a
serious condition.
Symptoms persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away.
Ask a doctor before use if you have
Persistent or chronic cough such as
occurs with smoking, asthma, chronic
bronchitis, or emphysema.
Cough that is accompanied by
excessive phlegm (mucus) or fever
Stop use and ask a doctor if
Cough persists for more than 1 week,
tends to recur, or is accompanied by a
fever, rash, or persistent headache. A
persistent cough may be a sign of a
serious condition.
Symptoms persist or worsen.
Other information
Do not use if seal is missing or broken.
Store in a cool, dry place.
Contents may not fill package in order to accommodate required labeling. Please rely on stated quantity
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as
recommended by your healthcare
practitioner.
Children (under 12 years)
Take under the direction of your
healthcare practitioner.
Uses
For the temporary relief of symptoms associated with minor respiratory problems.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as
recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.