PUREFORCE- benzalkonium chloride solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
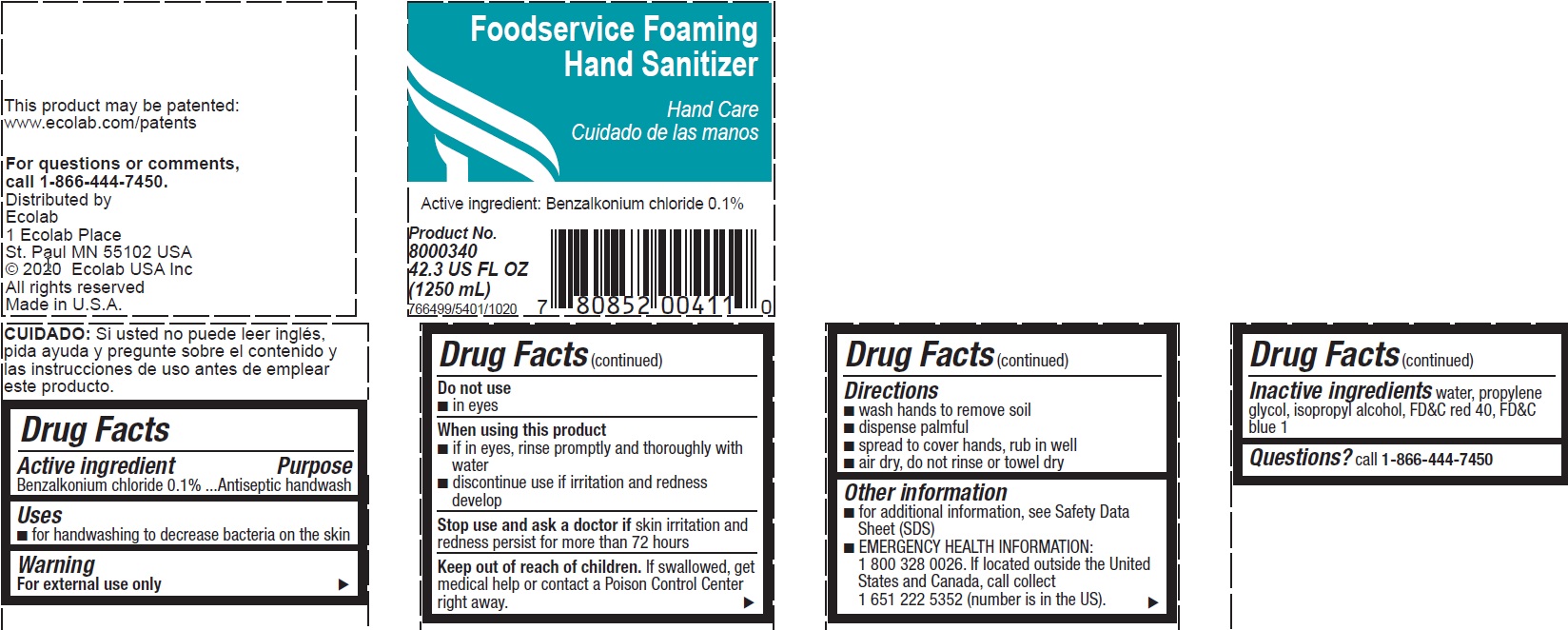
Benzalkonium chloride, 0.1%
Purpose
Antiseptic handwash
Uses
- For handwashing to decrease bacteria on the skin
Warnings
For external use only
When using this product
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
Stop use and ask a doctor if skin irritation or redness occurs for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wash hands to remove soil
- Dispense palmful
- Spread to cover hands, rub in well
- Air dry, do not rinse or towel dry
Other Information
- For additional information, see Safety Data Sheet (SDS)
- EMERGENCY HEALTH INFORMATION: 1 800 328 0026. If located outside the United States and Canada, call collect 1 651 222 5352 (number is in the US).
Inactive ingredients water, propylene glycol, isopropyl alcohol, FD&C red 40, FD&C blue 1
Questions? call 1-866-444-7450
Principal Display Panel and Representative Label
Foodservice Foaming
Hand Sanitizer
Hand Care
Active ingredient: Benzalkonium Chloride 0.1%
Product No.
8000340
42.3 US FL OZ (1250 mL)
766499/5401/1020
For questions or comments,
call 1-866-444-7450.
Distributed by
Ecolab
1 Ecolab Place
St Paul MN 55102 USA
© 2020 Ecolab USA Inc
All rights reserved
Made in U.S.A.
Ecolab Inc.
