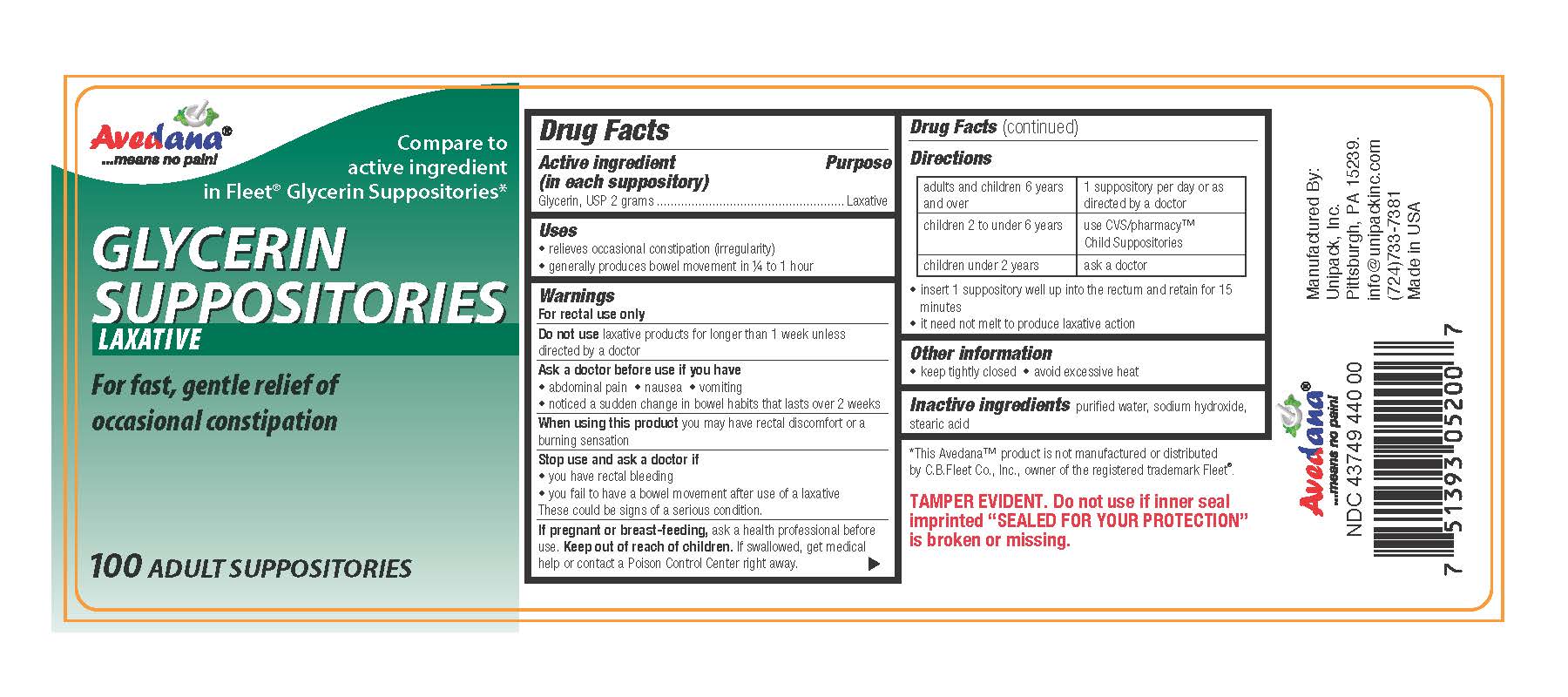
Active Ingredient
Active Ingredient Purpose
(in each suppository)
Glycerin, USP 2 grams ............................................Laxative
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 1/4 to 1 hour
Ask a doctor before use if you have
Ask a doctor before use if you have
- abdominal pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
When using this product
When using this product you may have rectal discomfort or a burning sensation
Stop use and ask a doctor if
Stop use and ask a doctor if
- you have rectal bleeding
- you fail to have a bowel movement after use of a laxative
These could be signs of a serious condition.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.