Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
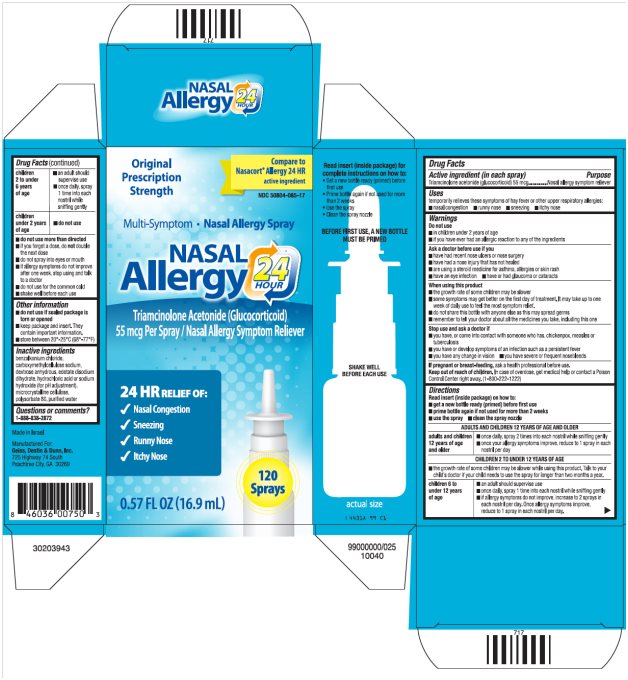
Uses
temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
- ■
- nasal congestion
- ■
- runny nose
- ■
- sneezing
- ■
- itchy nose
Warnings
Do not use
- ■
- in children under 2 years of age
- ■
- if you have ever had an allergic reaction to any of the ingredients
Ask a doctor before use if you
- ■
- have had recent nose ulcers or nose surgery
- ■
- have had a nose injury that has not healed
- ■
- are using a steroid medicine for asthma, allergies or skin rash
- ■
- have an eye infection
- ■
- have or had glaucoma or cataracts
When using this product
- ■
- the growth rate of some children may be slower
- ■
- some symptoms may get better on the first day of treatment. It may take up to one week of daily use to feel the most symptom relief.
- ■
- do not share this bottle with anyone else as this may spread germs
- ■
- remember to tell your doctor about all the medicines you take, including this one
Stop use and ask a doctor if
- ■
- you have, or come into contact with someone who has, chickenpox, measles or tuberculosis
- ■
- you have or develop symptoms of an infection such as a persistent fever
- ■
- you have any change in vision
- ■
- you have severe or frequent nosebleeds
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
Read insert (inside package) on how to:
- ■
- get a new bottle ready (primed) before first use
- ■
- prime bottle again if not used for more than 2 weeks
- ■
- use the spray
- ■
- clean the spray nozzle
|
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER |
|
|
adults and children 12 years of age and older |
|
|
CHILDREN 2 TO UNDER 12 YEARS OF AGE |
|
|
|
|
children 6 to under 12 years of age |
|
|
children 2 to under 6 years of age |
|
|
children under 2 years of age |
|
- ■
- do not use more than directed
- ■
- if you forget a dose, do not double the next dose
- ■
- do not spray into eyes or mouth
- ■
- if allergy symptoms do not improve after one week, stop using and talk to a doctor
- ■
- do not use for the common cold
- ■
- shake well before each use
Other information
- ■
- do not use if sealed package is torn or opened
- ■
- keep package and insert. They contain important information.
- ■
- store between 20°-25°C (68°-77°F)
Inactive ingredients
benzalkonium chloride, carboxymethylcellulose sodium, dextrose anhydrous, edetate disodium dihydrate, hydrochloric acid or sodium hydroxide (for pH adjustment), microcrystalline cellulose, polysorbate 80, purified water
Package/Label Principal Display Panel
NASAL Allergy 24 HOUR Carton Text
Original Prescription Strength
Compare to Nasacort® Allergy 24 HR active ingredient
NDC 50804-085-17
Multi-Symptom ·Nasal Allergy Spray
NASAL
Allergy 24 HOUR
Triamcinolone Acetonide (Glucocorticoid)
55 mcg Per Spray/Nasal Allergy Symptom Reliever
24 HR RELIEF OF:
- Nasal Congestion
- Sneezing
- Runny Nose
- Itchy Nose
120 Sprays
0.57 FL OZ (16.9 mL)