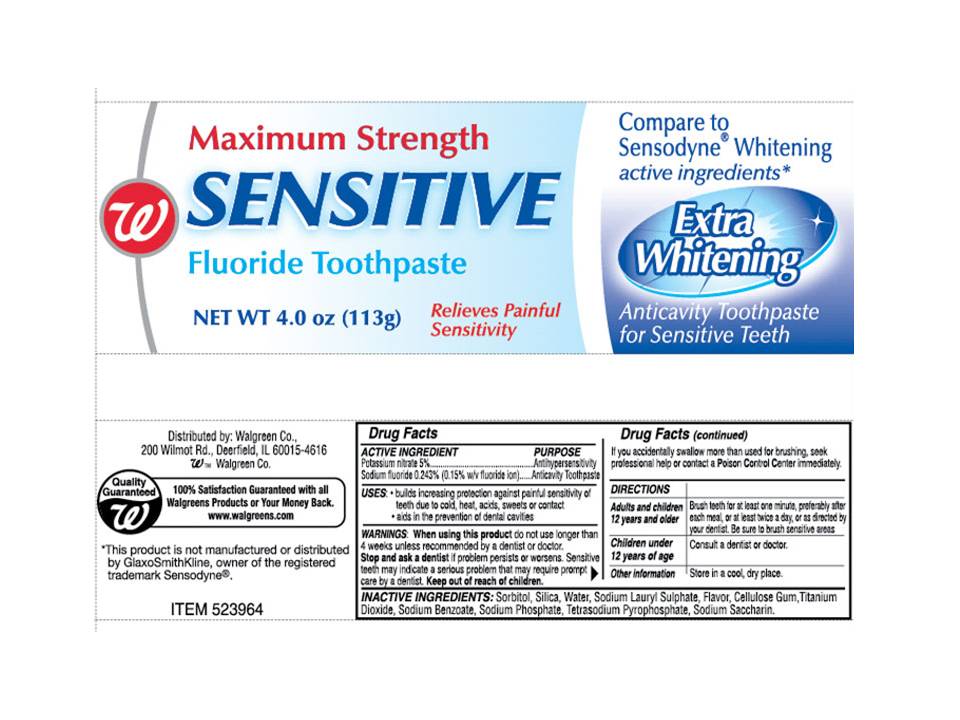
Active Ingredients
Potassium nitrate 5%........................................... Antihypersensitivity
Sodium Fluoride 0.243% (0.15% w/v fluoride ion).... Anticavity toothpaste
USES
- Builds increasing protection against painful sensitivity of teeth due to cold, heat, acids, sweets or contact
- Aids in the prevention of dental cavities
When using this product do not use longer than 4 weeks unless recommended by a dentist or doctor.
Stop and ask a dentist if problems persists or worsens. Sensitive teeth may indicate a serious problem that may require prompt care by a dentist.
Keep out of reach of children If you accidentally swallow more that used for brushing seek professional help or contact a Poison Control Center immediately.
Directions
Adults and children 12 years and older Brush teeth for at least one minute, preferably after each meal, or at least twice a day or as directed by your dentist. Be sure to brush sensitive areas.
Children under 12 years of age Consult a dentist or doctor
Other informationStore in a cool, dry place.