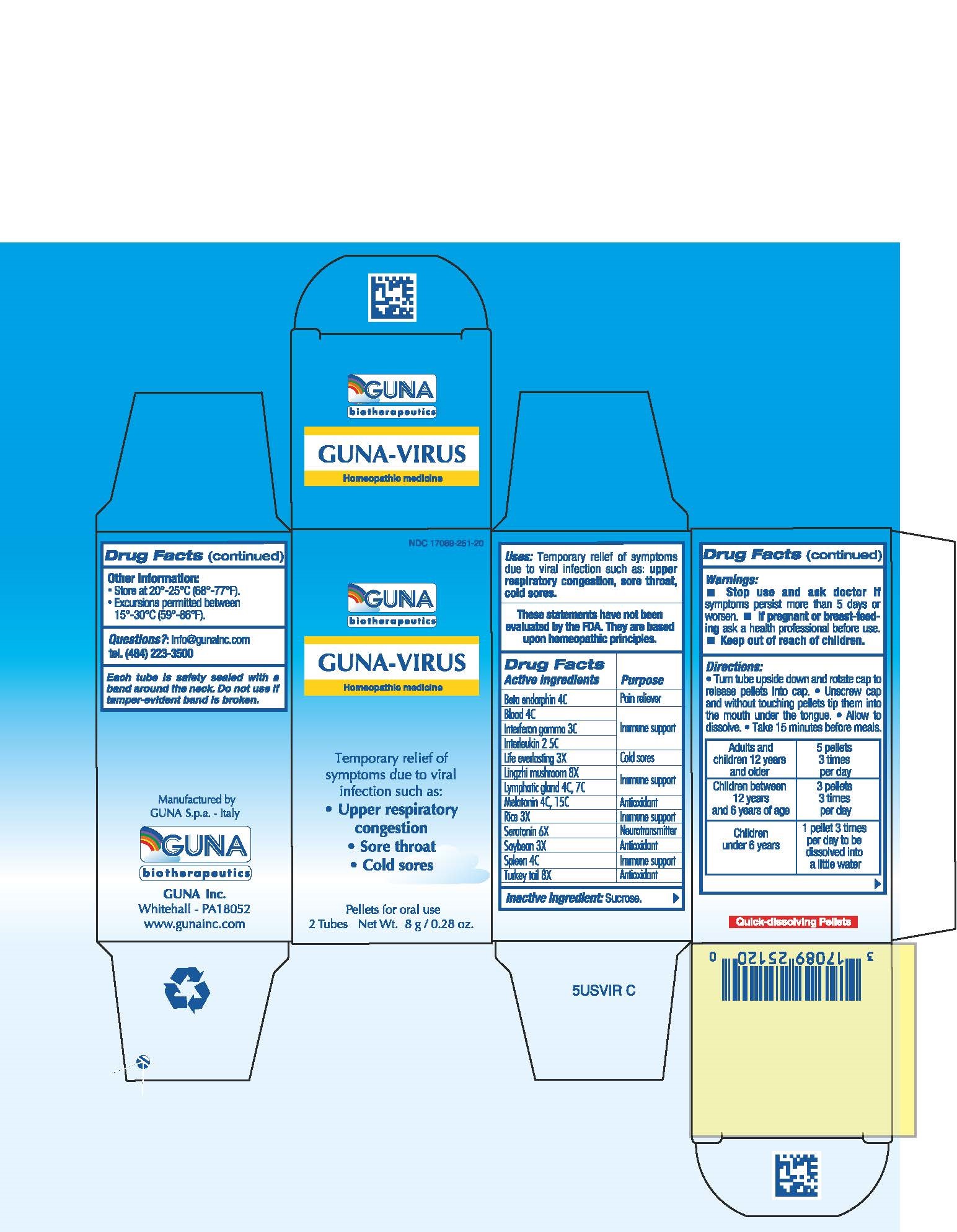
ACTIVE INGREDIENTS/PURPOSE
BETA ENDORPHIN 4C PAIN RELIEVER
BLOOD 4C IMMUNE SUPPORT
INTERFERON GAMMA 3C IMMUNE SUPPORT
INTERLEUKIN 2 5C IMMUNE SUPPORT
LIFE EVERLASTING 3X COLD SORES
LING CHIN MUSHROOM 8X IMMUNE SUPPORT
LYMPHATIC GLAND 4C 7C IMMUNE SUPPORT
MELATONIN 4C 15C ANTIOXIDANT
RICE 3X IMMUNE SUPPORT
SEROTONIN 6X NEUROTRANSMITTER
SOYBEAN 3X ANTIOXIDANT
SPLEEN 4C IMMUNE SUPPORT
TURKEY TAIL 8X ANTIOXIDANT
USES
Temporary relief of symptoms due to viral infection such as:
- Upper respiratory congestion
- Sore throat
-
Cold sores
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days or worsen.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children.
DIRECTIONS
- Turn tube upside down and rotate cap to release pellets into cap.
- Unscrew cap and without touching pellets tip them into the mouth under the tongue.
- Allow to dissolve
- Take 15 minutes before meals.