Use - After Bite Wipe
Temporarily protects and helps relieve minor skin irritation and itching due to - insect bites and stings, poison ivy, oak or sumac
Keep Out of Reach of Children - After Bite
Keep out of reach of children: if swallowed, get medical help or contact a Poison Control Center right away
Stop Use - After Bite Wipe
Stop use and ask a doctor if: Condition worsens, symptoms last more than 7 days or clear up and occur again within a few days
Directions - After Bite Wipe
Adults and Children 2 years and older - dab directly on bite or sting, rub gently and reapply as needed
Children under 2 years - ask a doctor
When Using - Aspirin
Reye's Syndrome: Children and teenagers who have or are recovering from chicken pox or Flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy Alert: Aspirin may cause a severe allergic reaction which may include: hives, skin reddening, facial swelling, rash, asthma (wheezing), blisters or shock. If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: this contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you: are age 60 or older; have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug; take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others), have 3 or more alcoholic drinks everyday while using this problem, take more for a longer time than directed.
Do not use if you have ever had an allergic reaction to any other pain reliever/fever reducer; right before or after heart surgery, if you are taking a prescription drug for gout, diabetes or arthritis.
Ask a doctor before use if: stomach bleeding warning applies to you, you have a history of stomach problems such as heartburn, you have high blood pressure, heart disease, liver cirrhosis or kidney disease, you are taking a diuretic.
Ask a Doctor or Pharmacist before use if you are: under a doctor's care for any serious condition, taking any other drug.
When using this product: take with food or milk if stomach upset occurs
Stop use and Ask a Doctor if: you experience any of the following signs of stomach bleeding, you feel faint, vomit blood, have bloody or black stools, have stomach pain that does not get better, gets worse or lasts more than 10 days, fever gets worse or lasts more than 3 days, you have difficulty swallowing, if ringing in the ears or loss of hearing occurs, redness or swelling is present in painful areas, or any new symptoms appear.
If pregnant or Breastfeeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless directed to do so by a doctor because it may cause problems in the unborn child or complications during deliver.
Keep out of reach of Children. In case of overdose, get medical help or contact a poison control center right away.
Directions - Aspirin
Do not use more than directed - the smallest effective dosage should be used. Drink a full glass of water with each dose. Do not take longer than 10 days, unless directed by a doctor.
Adults and Children (12 years and older): take 1 or 2 tablets with water every 4 hours as needed. Do not take more than 12 tablets in 24 hours or as directed as a doctor.
Children under 12 years: Do not give to children under 12 years of age
Avoid excessive heat and humidity, do not use any open or torn packets.
Use - Aspirin
Temporariy relieves minor aches and pains associated with: headache, muscular aches, minor arthritis pain, backache, common cold, toothache, menstrual cramps, temorarily reduces fever.
Use - Ibuprofen
Temporarily relieves minor aches and pains due to: the common cold, headache, toothache, muscular aches, backache, minor pain of arthritis, menstrual cramps. Temporarily reduces fever.
Warnings and Precautions - Ibuprofen
Allergy alert: ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include: shock, facial swelling, asthma (wheezing), rash, skin reddening, blisters, and hives, if an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: this product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you: are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinner (anticoagulant) or steroid drug, take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed.
Do not use if you have ever had an allergic reaction to any other pain reliever/fever reducer, fight before or after heart surgery.
Ask a doctor before use if stomach bleeding warning applies to you, you have had a history of stomach problems such as heartburn, you have a high blood pressure, heart disease, liver cirrhosis, or kidney disease, you are taking a diuretic.
Ask a doctor before use if you are taking any other drug containing NSAID (prescription or nonprescription), taking aspirin for heart attack or stroke, because Ibuprofen may decrease this benefit of aspirin, taking any other drug.
When using this product take with food or milk if stomach upset occurs.
Stop use and ask a doctor if you experience any of the following signs of stomach bleeding, feeling faint, vomit blood, have bloody or black stools, have stomach pain that does not get better, pain gets worse or lasts more than 10 days, fever gets worse or lasts more than 3 days, redness or swelling is present in the painful area, any new symptoms appear.
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a poison control center right away.
Store at controlled room temperature, avoid excessive heat 40 degrees Celsius (104 degrees Fahrenheit); temper evident sealed packets, do not use any opened or torn packets.
Do not use more than directed, the smallest effective dose should be used, do not take longer than 10 days, unless directed by a doctor. Adults and Children (12 years and older): take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 3 tablets may be use. Do not exceed 6 tablets in 24 hours, unless directed by a doctor. Children under 12 years: do not give children under 12 years of age.
Uses - APAP
Temporary relief of minor aches and pains associated with: common cold, headache, toothache, muscular aches, backache, arthritis, menstrual cramps, and reduction of fever
Warnings and Precautions - APAP
Liver Warning: This product contains acetaminophen.
Severe liver damage may occur if: adult takes more than 12 tablets in 24 hours, which is the maximum daily amount, child takes more than 5 doses in 24 hours, taken with other drugs containing acetaminophen, adult has 3 or more alcoholic drinks ever day while using this product.
Do not use with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist, for more than 10 days for pain unless directed by a doctor, for more than 3 days for fever unless directed by a doctor.
Ask a doctor before use if the user has liver disease.
Ask a doctor or pharmacist before use if the user is taking the blood thinning drug warfarin.
Stop use and ask a doctor if: symptoms do not improve, pain gets worse or lasts more than 10 days, fever gets worse or lasts more than 3 days, new symptoms occur, redness or swelling is present, a rare sensitivity reaction occurs.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of accidental overdose, contact a doctor or Poison Control Center immediately. Prompt medical attention is critical for adults as well for children even if you do not notice any signs or symptoms. Do not exceed recommended dosage.
Store at 59-86 degrees F (15-30 degrees C), tamper evident sealed packets, do not use any open or torn packets.
Directions - APAP
Adult and Children (12 years and older) take 2 tablets every 4 to 6 hours as needed. Do not take more than 12 tablets in 24 hours.
Children 6-11 years take 1 tablet every 4 to 6 hours as needed. Do not take more than 5 tablets in 24 hours.
Childen under 6 do not use this product
Use - Diphenhydramine
Temporarily relieves these symptoms due to hay fever or other respiratory allergies: runny nose, sneezing, itching nose or throat, itchy-watery eyes.
Temporarily relieves these symptoms due to the common cold: runny nose, sneezing
Warnings and Precautions - Diphenhydramine
Do not use to make a child sleepy or with any other product containing diphenhydramine, even one that is used on skin.
Ask a doctor before use if you have: a breathing problem such as emphysema or chronic bronchitis, difficulty in urination due to enlargement of the prostate gland, or glaucoma.
Ask a doctor pharmacist before you use if you are taking sedatives or tranquilizers.
When using this product: marked drowsiness may occur, avoid alcoholic beverages, alcohol, sedatives and tranquilizers may increase the drowsiness effect, use caution when driving a motor vehicle or operating machinery, excitability may occur, especially in children.
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of Children. In case of overdose, contact a physician or poison control center immediately.
Each caplet may contain calcium 25mg. Store at room temperature 59-86 degrees F (15-30 C), protect from light and use by expiration date on packet. Tamper-evident sealed packets, do not use any opened or torn packets.
Directions - Diphenhydramine
Do not use more than directed.
Adults and Children (12 Years and older) - take 1 to 2 caplets every 4 to 6 hours as needed. Do not take more than 12 caplets in 24 hours, or as directed by a doctor.
Do not give to children under 12 years of age.
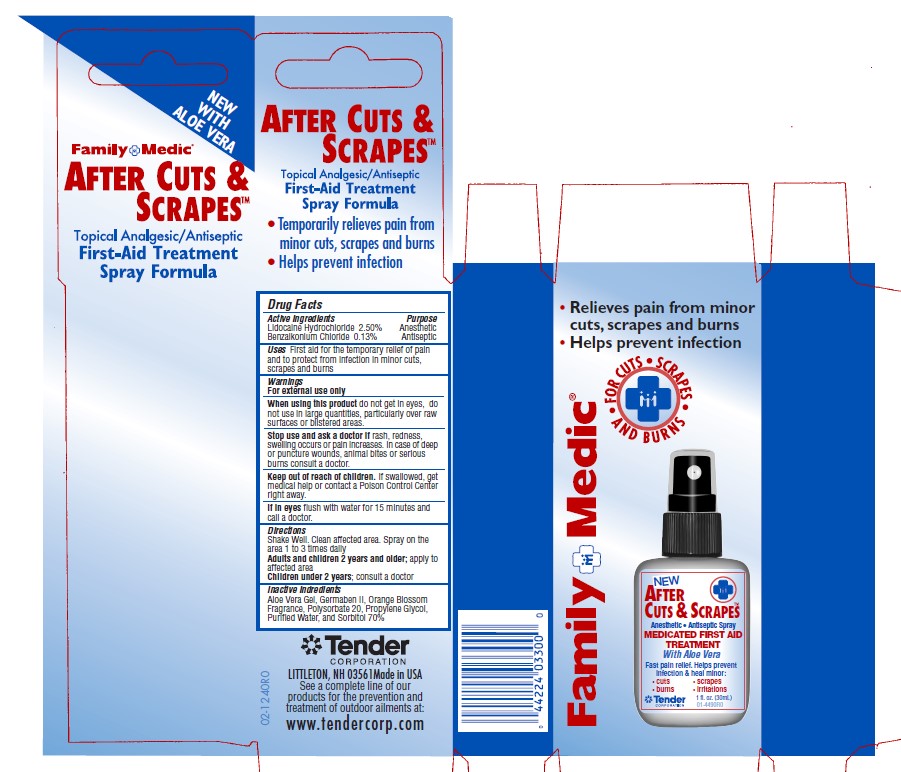
Uses - After Cuts and Scrapes
First aid for the temporary relief of pain and to protect from infection in minor cuts, scrapes and burns.
Warnings and Precautions - After Cuts and Scrapes
For External Use Only.
When using this product: do not get into eyes, do not use in large quantities, particularly over raw surfaces or blistered areas.
Stop use and ask a doctor if rash, redness or swelling occurs or pain increases. In case of deep puncture wounds, animal bites or serious burns consult a doctor.
If swallowed, get medical help or contact a poison control center right away.
If in eyes, flush with water for 15 minutes and call a doctor.
Keep out of Reach of Children.
Directions - After Cuts and Scrapes
Shake Well. Clean affected area. Spray on the area 1 to 3 times daily. Adults and children 2 years and older, apply to affected area. Children under 2 years, ask a doctor.
Use - After Burn
For the temporary relief of pain due to minor burns, sunburn, minor cuts, scrapes or minor skin irritations
Warnings and Precautions - After Burn
For external use only.
When using this product: avoid contact with eyes, do not use in large quantities, particularly over raw surfaces or blistered areas, do not use longer than 1 week unless directed by a doctor, or in case of deep puncture wounds, animal bites or serious burns, consult a doctor.
Stop use and ask a doctor if conditions or symptoms persist for more than 7 days or clear up and occur again within a few days.
Keep out of Reach of Children.
If swallowed, get medical help or contact a poison control center right away.
If in eyes, flush with water for 15 minutes and call a doctor
Adults and Children 2 years and older, apply small amount to affected area, not more than 3 times daily.
Children under 2 years, consult a doctor.