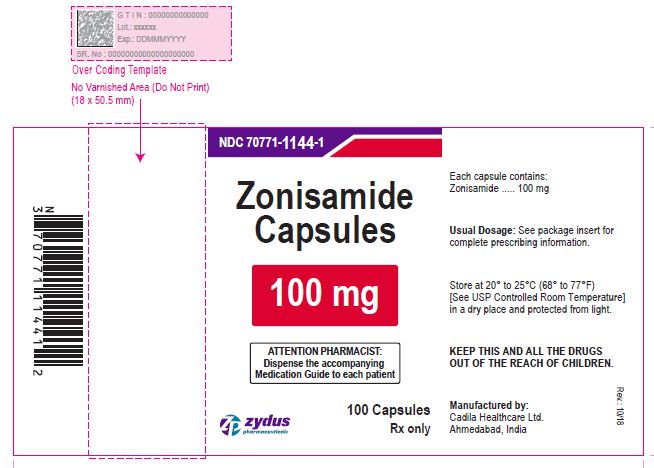
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1142-1 in bottle of 100 Capsules
Zonisamide Capsules, 25 mg
Rx only
100 Capsules
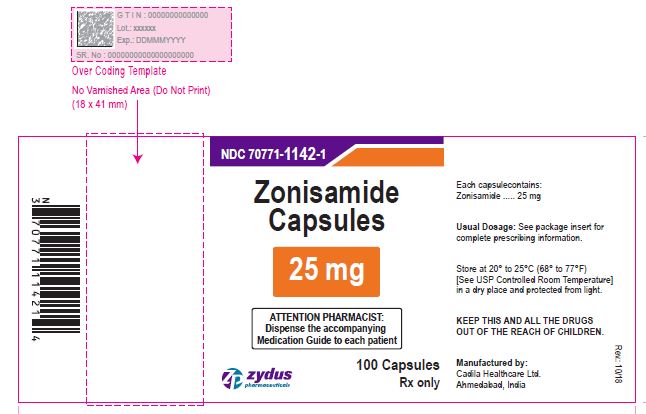
NDC 70771-1143-1 in bottle of 100 Capsules
Zonisamide Capsules, 50 mg
Rx only
100 Capsules
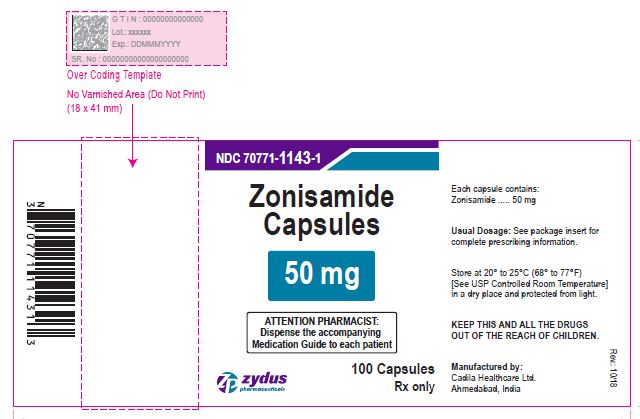
NDC 70771-1144-1 in bottle of 100 Capsules
Zonisamide Capsules, 100 mg
Rx only
100 Capsules