LAVIV - azficel-t injection, suspension
Fibrocell Technologies, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LAVIVTM (azficel-T) safely and effectively. See full prescribing information for LAVIVTM.
LAVIVTM. (azficel-T) Suspension for Intradermal Injection. Initial U.S. Approval: 2011 INDICATIONS AND USAGELAVIVTM (azficel-T) is an autologous cellular product indicated for improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults. (1)
DOSAGE AND ADMINISTRATION
For autologous intradermal injection only
DOSAGE FORMS AND STRENGTHSA single vial of LAVIV contains approximately 18 million autologous fibroblasts in a 1.2 milliliters suspension, sufficient to administer 1 milliliter of product. (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions, occurring in ≥ 1% of patients who received LAVIV, were injection-site redness, bruising, swelling, pain, hemorrhage, edema, nodules, papules, irritation, dermatitis, and pruritus. (6) Adverse reactions occurring in fewer than 1% of trial subjects were acne, facial or eyelid edema, hypersensitivity or decreased skin sensation at the injection site, post-procedural discomfort (headache, toothache, and jaw pain), herpes labialis, hyperpigmentation at the injection site, injection-site ischemia, basal cell cancer, and leukocytoclastic vasculitis. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Fibrocell at 484-713-6000 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSPregnancy: No human or animal data. Use only if clearly needed. (8.1) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 6/2011 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
LAVIVTM is an autologous cellular product indicated for improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults.
The safety and efficacy of LAVIV for areas other than the nasolabial folds have not been established.
The efficacy of LAVIV beyond six months has not been established.
2 DOSAGE AND ADMINISTRATION
For autologous intradermal injection only
Only healthcare providers who have completed a Fibrocell-approved training program should administer LAVIV.
2.1 Dosage
Inject LAVIV at 0.1 milliliter per linear centimeter into the nasolabial fold wrinkles. The recommended treatment regimen is three treatment sessions, administering up to 2 milliliters (2 vials) of LAVIV per session, at 3-6 week intervals.
2.2 Preparation
- Confirm that the unique patient identifier on the LAVIV vial matches the identity of the patient who will receive the LAVIV injections.
- Allow the LAVIV vial(s) to reach room temperature before use.
- Examine vial(s) for leaks and for any evidence of damage or contamination.
- Gently invert each vial to re-suspend the product within the media. Tap the top of the vial to release any fluid retained in the top of the vial prior to opening the vial. DO NOT DILUTE THE PRODUCT.
- Before the injection, prepare a minimum of four sterile syringes and needles. Small unit syringes (e.g., 0.5 milliliter insulin syringes) are recommended for better injection control. A detachable larger bore needle (e.g., 21-gauge) should be used to withdraw product from the vials to minimize cell damage.
- Using aseptic technique, unscrew the vial cap and withdraw up to 0.5 milliliters from the vial into each syringe, noting the total volume. After a 21-gauge needle is used to withdraw LAVIV from the vial, the needle should be replaced with a 30-gauge needle prior to injection. Short, sharp needles (e.g., 30-gauge, half-inch needles) are recommended for better injection control and minimization of inflammation.
2.3 Administration
- Identify the areas to be injected and make sure the injection areas are free of cosmetics, hair or facial jewelry.
- Evaluate the need for topical anesthesia. If a topical anesthetic is administered, remove any topical anesthetic from the face prior to injection of LAVIV. DO NOT USE injectable local anesthetics.
- Clean the treatment area with an aseptic solution prior to injection.
- Place the patient in a comfortable position (e.g., recumbent) to facilitate proper injection angle.
- Inject LAVIV into the superficial papillary dermis at 0.1 milliliter per linear centimeter, using a 30-gauge needle. When the needle is inserted into the correct plane of the skin (i.e., along the line of each nasolabial fold wrinkle), the needle should be visible through the epidermis.
- Confirm intradermal injection by the appearance of blanching and a fluid bleb at the injection site. Avoid injecting LAVIV into the blood vessels, subcutaneously, or intramuscularly.
- Apply multiple injections as needed to cover the entire nasolabial fold wrinkle. Overlap injection areas slightly; otherwise, the last few millimeters of each injection site may receive no product. To prevent exudation of LAVIV from the injection site, make sure that the end of the needle is inserted slightly adjacent to the tract of the previous injection.
- After the injection, leave the treated area undisturbed. Do not rub, massage or compress the area. Apply a cold pack for 2-3 minutes. Do not place ice directly against the skin.
- Discard leftover LAVIV and injection materials as biohazardous waste.
- Counsel the patient on care of the injection site.
3 DOSAGE FORMS AND STRENGTHS
A single vial of LAVIV contains approximately 18 million autologous fibroblasts in a 1.2 milliliters suspension, sufficient to administer 1 milliliter of product.
4 CONTRAINDICATIONS
- Allogeneic use
If LAVIV is administered to a patient who is not the individual whose skin was used to produce the LAVIV, serious immunological reactions can occur. Each vial of LAVIV has a unique patient identifier to assist in ensuring that there is no mismatch.
- Severe Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, can occur in patients with known hypersensitivity to the ingredients in LAVIV. Do not use LAVIV in patients allergic to gentamicin, amphotericin, dimethyl sulfoxide (DMSO), or material of bovine origin.
- Active Infection
Injecting LAVIV into areas with skin infections can lead to local or systemic infection.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have occurred in patients treated with LAVIV.
5.2 Bleeding and Bruising
Injection-site bleeding and bruising can occur in patients treated with LAVIV. Patients taking aspirin, non-steroidal anti-inflammatory drugs (NSAIDS) or anticoagulants, as well as patients with coagulopathies, have a greater risk of severe bleeding or bruising. [See Drug Interactions (7)]
5.3 Vasculitis
Leukocytoclastic vasculitis has occurred following treatment with LAVIV. [See Adverse Reactions (6.1)]
5.5 Malignancy
Chemotherapeutic drugs may interfere with the function of LAVIV. Patients with malignancies requiring ongoing therapy should not use LAVIV. Furthermore, patients undergoing chemotherapy are often immunosuppressed, and therefore may be at increased risk of infection following the use of LAVIV. [See Immunosuppression (5.8)]
A case of basal cell carcinoma was reported near the injection site seven months following LAVIV treatment in clinical trials. While the etiology of the case is uncertain, LAVIV is not recommended for patients with a known history of skin cancer.
5.6 Keloid Formation
The use of LAVIV requires three post-auricular skin biopsies and multiple injections in the nasolabial folds. These procedures traumatize the skin and may lead to keloid or hypertrophic scar formation in susceptible individuals. While excessive scarring or keloid formation was not observed in either the post-auricular biopsy or the injected areas during the clinical trials, the study population included only four African-American subjects, and Fitzpatrick skin types were not recorded. LAVIV is not recommended for patients with a known history of keloids or hypertrophic scarring.
5.7 Genetic disorders
Disorders affecting dermal fibroblasts, formation of normal collagen matrices, or other skin components may cause an abnormal response to LAVIV. Thus, LAVIV is not recommended for patients with such genetic disorders as Ehlers-Danlos syndrome, achondroplasia, osteogenesis imperfecta, epidermolysis bullosa, Marfan syndrome, and ataxia-telangiectasia.
5.8 Immunosuppression and Autoimmune Disorders
Patients with active autoimmune disease or patients on immunosuppressant therapies may be more susceptible to infection and have difficulty healing following the use of LAVIV. LAVIV is an autologous cell product, and the safety and efficacy of LAVIV in patients with autoimmune disease are unknown.
5.9 Control of Infectious Disease
Patients undergoing the treatment procedures associated with LAVIV are not routinely tested for adventitious viruses. Therefore, healthcare providers should employ universal precautions when handling LAVIV or biopsy material.
5.10 Sterility Testing
LAVIV is shipped following a passing sterility test result for cryopreserved bulk material (Drug Substance) and a negative Gram stain test result on the final LAVIV drug product. Full sterility test results are not available for the LAVIV drug product prior to treatment for up to 14 days. If microbial contamination is detected after the product has been shipped, Fibrocell will notify the health care providers and recommend appropriate actions.
6 ADVERSE REACTIONS
The most common adverse reactions, occurring in ≥1% of subjects in clinical trials, were injection-site reactions, including redness, bruising, swelling, pain, hemorrhage, edema, nodules, papules, irritation, dermatitis, and pruritus.
Adverse reactions occurring in less than 1% of trial subjects were acne, facial or eyelid edema, hypersensitivity or decreased skin sensation at the injection site, post-procedural discomfort (headache, toothache, and jaw pain), herpes labialis, hyperpigmentation at the injection site, injection-site ischemia, basal cell cancer, and leukocytoclastic vasculitis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
The overall clinical trial safety database for LAVIV includes 508 subjects who received at least one treatment of LAVIV and 354 subjects who received a vehicle-control in seven clinical trials for treatment of facial wrinkles (a skin biopsy study is described separately). This total safety population included patients aged 20 to 79 years, of whom 92% were female and 92% were White. The average duration of observation in the safety population was approximately 12 months.
An integrated summary of the adverse reactions from the seven clinical trials is presented in Table 1. More than 80% of all adverse reactions were local and required no treatment. Eighty-six percent of all injection-site adverse reactions resolved within one week.
The adverse reactions to the vehicle (Table 1) should not be viewed as adverse reactions to a placebo but rather as reactions to a non-cellular component of LAVIV, or to the injection procedure, or to both.
|
* Number and percent of subjects with injection-site reactions |
||
| LAVIV (508 Subjects)
n (%)* | Vehicle (354 Subjects)
n (%)* |
|
| Any Injection-Site Reaction | 343 (67) | 144 (40) |
| Erythema | 81 (16) | 33 (9) |
| Bruising | 54 (11) | 48 (14) |
| Swelling | 69 (14) | 15 (4) |
| Pain | 31 (6) | 6 (2) |
| Hemorrhage | 13 (3) | 16 (5) |
| Edema | 22 (4) | 0 |
| Nodules | 20 (4) | 3 (<1) |
| Papules | 8 (2) | 3 (<1) |
| Irritation | 6 (1) | 1 (<1) |
| Dermatitis | 5 (1) | 2 (<1) |
| Pruritus | 5 (1) | 3 (<1) |
Adverse reactions occurring in fewer than 1% of trial subjects were acne, facial or eyelid edema, hypersensitivity or decreased skin sensation at the injection site, post-procedural discomfort (headache, toothache, and jaw pain), herpes labialis, hyperpigmentation at the injection site, injection-site ischemia, basal cell cancer, and leukocytoclastic vasculitis. In the skin biopsy study (described below), one subject was hospitalized for leukocytoclastic vasculitis, with lesions on his legs and trunk appearing nine days after product administration in his upper arm. These lesions resolved within 35 days after onset.
Skin Biopsy Study
A skin biopsy study was conducted to observe tissue responses and adverse reactions to LAVIV at the histological level. Twenty-nine subjects who had participated in a prior clinical trial of LAVIV, and who had sufficient quantities of autologous fibroblasts remaining, received up to three treatments of LAVIV in their upper arms at five-week intervals. Seven subjects received three treatments; 21 received two treatments; and one received only one treatment. Saline injections and non-treated areas in the contralateral arm were used as controls. The dose of LAVIV used in the skin biopsy study was the same as in the clinical trials (0.1 milliliter per linear centimeter of skin). The subjects and the two independent histopathology evaluators were blinded to treatment at each skin area.
Biopsies were taken from LAVIV- and saline-treated sites at 3 and 6 months after the last injection. Biopsies from untreated sites were taken only at 3 months. Histological evaluation was performed for all 29 subjects. At 3 months, histological examination showed inflammatory cell infiltration in 50% of LAVIV-treated sites as compared with 7% of placebo-treated or untreated sites. At 6 months, inflammatory cell infiltration was seen in 27% of the LAVIV-treated sites compared with 12% of placebo-treated sites. In all 29 subjects, there was no histological evidence of abnormal fibroblasts, significant scar formation, or abnormal organization of the extracellular matrix. There were no observed differences in epidermal/dermal thickness or cellularity between LAVIV-injected and placebo-injected skin samples.
7 DRUG INTERACTIONS
No drug interaction studies have been performed with LAVIV.
Patients taking aspirin, NSAIDS or anticoagulants may experience increased bruising or bleeding at biopsy and/or injection sites. Concomitant use of aspirin, NSAIDs or anticoagulants is not recommended. Decisions regarding continued use or cessation of anticoagulants should be made in consultation with the health care provider.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with LAVIV. It is also unknown whether LAVIV can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. LAVIV should be used in a pregnant woman only if clearly needed.
8.5 Geriatric Use
Clinical studies of LAVIV did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. [See Clinical Studies (14)]
11 DESCRIPTION
LAVIV is an autologous cellular product composed of fibroblasts suspended in Dulbecco's Modified Eagle's Medium (DMEM) without phenol red. Dermal fibroblasts from post-auricular skin biopsy tissue are aseptically expanded using standard tissue-culture procedures until sufficient cells for three doses are obtained. Cells are then cryopreserved in a protein-free solution containing DMSO. When the patient administration is scheduled, cells are thawed, washed, and shipped to the clinic. A final sterility test is initiated prior to shipping, but the result will not be available for up to 14 days. A passing sterility culture result from the test performed on the cryopreserved bulk material (Drug Substance) and a negative Gram stain test result on the final LAVIV drug product are required for release of a product for shipping.
14 CLINICAL STUDIES
The effectiveness of LAVIV was demonstrated in two identically-designed, multi-center, randomized, double-blind, vehicle-controlled studies. The study population consisted of subjects with moderate to severe bilateral nasolabial fold wrinkles. A total of 421 subjects, aged 23 to 81 years, were randomized to receive LAVIV (n=210) or vehicle-control (n=211). In both studies, the major demographic features were similar between LAVIV and vehicle-control groups. Subjects in Study One (n=203) were predominantly female (90%), White (95%), and had an overall mean age of 56.7 years. Subjects in Study Two (n=218) were predominantly female (91%), White (89%), and had an overall mean age of 54.6 years.
Each subject underwent three post-auricular skin punch biopsies to obtain skin tissue to generate LAVIV. Subjects whose biopsy samples met acceptance criteria were randomized in a 1:1 ratio to receive either LAVIV or vehicle-control (DMEM without phenol red). Biopsy samples for subjects assigned to receive LAVIV were provided to the manufacturer for culture and processing. The process for production of LAVIV takes approximately 11 to 22 weeks. Study agent (either LAVIV or vehicle-control) was then administered intradermally to nasolabial fold wrinkles on both sides of the face at a dose of 0.1 milliliter per linear centimeter, up to 2 milliliters (1-2 x107 cells/milliliter) per each treatment session. A total of three separate treatment sessions occurred at intervals of 5±1 weeks.
Since LAVIV must be grown from subjects' own skin biopsies, the manufacturing process may not yield sufficient quantities of fibroblasts. Due to product manufacturing failure, 6.2% of subjects randomized to receive LAVIV in the two trials did not receive any LAVIV. In addition, 5.7% of subjects randomized to receive LAVIV had insufficient quantities of LAVIV to complete the planned three treatment sessions. A repeat biopsy was required in 1.5% of subjects as a result of shipping errors.
For both clinical trials, the co-primary efficacy outcomes were the proportion of subjects with a two-point improvement from baseline in the appearance of the nasolabial fold wrinkles at six months after the third treatment session. The outcomes were assessed with live evaluations performed independently by subjects and by evaluating physicians. Both subjects and evaluators were blinded to treatment assignment. To maintain the blinding, the injector and evaluator for any given subject were different investigators. Subjects assessed their nasolabial fold wrinkles on a five-point Subject Wrinkle Assessment scale that ranged from -2 to +2. The evaluating physicians used a six-point Evaluator Wrinkle Severity Assessment scale that ranged from 0 to 5 (Lemperle scale). Evaluation with the Lemperle scale was aided by a photoguide, which correlated wrinkle appearance with a specific numerical score.
Table 2 shows the efficacy results based on the intent-to-treat population, which included all randomized subjects. When subjects assessed themselves, 57% (Study One) and 45% (Study Two) of subjects receiving LAVIV achieved a 2-point improvement in the appearance of their nasolabial fold wrinkles compared with 30% (Study One) and 18% (Study Two) of subjects receiving vehicle-control. When assessed by evaluating physicians, 33% (Study One) and 19% (Study Two) of subjects receiving LAVIV achieved a 2-point improvement in their nasolabial fold wrinkles, compared with 7% of subjects receiving vehicle-control in both trials.
Efficacy beyond six months after the third administration has not been established. No clinical studies have been conducted to evaluate the efficacy of repeating treatment(s) beyond six months.
|
*The comparison of LAVIV vs. vehicle-control is based on Cochran-Mantel-Haenszel test stratified by study site. |
||||||
| Clinical Studies | Subject Wrinkle Assessment | Physician Wrinkle Assessment | ||||
| LAVIV | Vehicle | p-value* | LAVIV | Vehicle | p-value* | |
| Study One | 57% (57/100) | 30% (31/103) | 0.0001 | 33% (33/100) | 7% (7/103) | < 0.0001 |
| Study Two | 45% (50/110) | 18% (19/108) | < 0.0001 | 19% (21/110) | 7% (8/108) | 0.0075 |
Geriatric
Clinical studies did not include sufficient numbers of subjects aged 65 years and older to determine whether their responses to LAVIV treatment differ from those of younger subjects. Of the 421 subjects in the two efficacy trials, 71 (17%) were ≥ 65 years old. The overall responder rates in geriatric subjects were lower and less consistent than in subjects younger than 65 years of age.
16 HOW SUPPLIED / STORAGE AND HANDLING
LAVIV is intended solely for autologous use.
LAVIV is supplied in two vials, each containing approximately 18 million cells in 1.2 milliliters. The vials are packaged together in a tamper-evident biohazard bag and shipped at 2-8°C (36-46°F) inside a temperature-controlled shipping container.
The manufacturing process for LAVIV takes approximately 11-22 weeks after receipt of the patient's biopsy samples by the manufacturer. Fibrocell will notify the clinic when each patient's treatment may be scheduled.
Storage and Handling
- Employ universal precautions when handling LAVIV. Patients undergoing procedures associated with LAVIV are not routinely tested for adventitious viruses.
- DO NOT FREEZE, sterilize, or incubate LAVIV, as this may result in inactivation of the product.
- Protect LAVIV from exposure to sunlight.
- Inspect the packaging and vials for damage. LAVIV should not be used if the packaging, injection vial(s), or seal(s) are damaged.
- Store each vial on its side at 2-8°C (36-46°F) to minimize viscosity.
- Remove vials from the refrigerator 15-30 minutes before use to allow them to reach room temperature.
- Use product prior to the expiration date and time printed on the vial.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Prior to initiating treatment with LAVIV, the healthcare provider should
- Ask the patient about any history of skin cancer, keloids, scarring, or immune problems.
- Discuss the biopsy process and ask the patient to report any persistent symptoms related to the biopsy procedure.
- Manage expectations by telling the patient that
- The effects of LAVIV are not immediate, but may appear over the course of the three-treatment regimen.
- Sometimes the biopsies will not produce enough viable cells to manufacture enough LAVIV for injection. In such cases, the patient will be notified and may be offered the opportunity to repeat the procedure.
Following treatment with LAVIV, provide post-injection instructions including the following
- Do not apply ice to the face. A cold compress may be applied for 2-3 minutes at a time, if needed to ease swelling or discomfort.
- Do not wash the site of injections for at least 24 hours.
- Do not scrub, rub or manipulate the treatment area for at least 72 hours.
- Do not apply any products (e.g., make-up, facial creams, sunscreen) to the treatment area for 72 hours.
- Expect mild to moderate redness, swelling, puffiness, or bruising following injections.
- Call the doctor or emergency department for any persistent or unexpected side effects.
Manufactured and Distributed by
Fibrocell Technologies, Inc.
405 Eagleview Blvd.
Exton, PA 19341
T: 484-713-6000
F: 484-713-6001
www.fibrocellscience.com
U.S. License No. 1818
LAVIVTM is a registered trademark of Fibrocell Science, Inc., Exton, PA
LAVIVTM, autologous human fibroblast cells in suspension for injection, is covered by the following U.S. patent: U.S. Pat. No. 5591444.
FDA-Approved Patient Labeling
LAVIVTM(azficel-T)
(pronounced lah-VEEV)
This leaflet is designed to help you understand LAVIVTM. This leaflet does not take the place of talking with your healthcare provider about LAVIV. If you have questions about LAVIV, talk to your healthcare provider.
What is the most important information I should know about LAVIV?
LAVIV is made especially for you from your own skin cells. Your healthcare provider will check to see that the cells that come back from the manufacturer are yours. Using someone else's cells can cause a serious reaction. Do not let anyone else use your LAVIV.
The process for making LAVIV uses antibiotics (amphotericin and gentamicin), bovine serum (from cattle), and dimethyl sulfoxide (DMSO). If you are allergic to any of these, tell your healthcare provider.
Do not use LAVIV if you have a skin infection on your face because LAVIV treatment can make the infection worse. Talk to your healthcare provider if you have any other infection.
What is LAVIV?
LAVIV is made from your own skin cells, which are used to improve the appearance of wrinkles that go from the sides of your nose to the corners of your mouth (called nasolabial folds). LAVIV is injected into your face using a small needle.
Who should not get LAVIV?
You should NOT get LAVIV if you have any of the following problems or conditions:
- Allergy to the antibiotics gentamicin or amphotericin, DMSO, or to things made from cattle (bovine).
- Skin infections in the face.
What should I tell my healthcare provider before getting LAVIV?
Your health care provider will help you to decide whether you are a candidate for LAVIV and may help you avoid some of the adverse reactions from LAVIV. Before getting LAVIV, tell your healthcare provider if you have any of the following medical problems:
- Allergic reactions to any drugs or food
- Bleeding disorders or take blood-thinning medicines like aspirin, ibuprofen, or coumadin
- Keloids or excessive scarring
- Skin cancer or any malignancy
- Genetic disorders affecting your skin
- Immune problems or take medicines that affect your immune system
- Any other illness or medical problem
How will I get LAVIV?
Your healthcare provider will take three small skin samples (called biopsies) from behind your ears and send them to the manufacturer. Certain cells, called fibroblasts, are grown from the samples. This takes about three to six months.
The cells (LAVIV) are sent back to your healthcare provider, who will inject them into your nasolabial fold wrinkles using a small needle.
You usually get LAVIV in three treatment sessions about 3-6 weeks apart.
It is very important that you arrive on time for your treatment sessions. If you miss a treatment session, your LAVIV cells will expire and must be thrown out. Your healthcare provider will work with you to schedule a new treatment session.
There is a chance that your skin samples will not make enough cells to use. In this case, your doctor may ask you if you wish to try biopsy again.
What should I avoid while I am getting LAVIV treatments?
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription drugs (like aspirin, vitamins, and dietary supplements). Your healthcare provider will advise you about the use of these medicines during LAVIV treatment.
- People who have bleeding disorders or who take blood thinning medicines may have more bleeding with LAVIV treatment.
- People with immune problems or who take medicines that affect the immune system can get infection or have healing problems with LAVIV treatment.
Expect some redness, swelling, puffiness, pain or bruising following LAVIV treatments. You can help yourself by doing the following:
- Do not rub, scrub, or massage the injection site for at least 72 hours.
- Apply a cold compress for 2-3 minutes at a time. DO NOT apply ice to the face.
- Do not wash the site of injections for at least 24 hours.
- Do not use make-up, facial creams, sunscreen, or other skin products on the face for 72 hours.
What are the possible or reasonably likely side effects of LAVIV?
The most common side effects are at the injection-site, including
- Redness
- Bruising
- Swelling
- Pain
- Bleeding
- Lumps
- Irritation
- Itchiness
Tell your healthcare provider or call the emergency department right away if you have
- Difficulty breathing, trouble swallowing, rash, hives, or severe redness and swelling, because these may be signs of a serious allergic reaction.
- Fever over 100ºF, redness, warmth, or pain at the injection or biopsy sites lasting for more than three days, because these may be signs of serious infection.
These are not all the possible side effects of LAVIV. Tell your health provider about any side effects that concern you. You may report side effects to FDA at 1-800-FDA-1088.
What are the ingredients in LAVIV?
LAVIV is made from your own skin cells placed in a mixture of water and salts.
Antibiotics (amphotericin and gentamicin), bovine serum (from cattle) and dimethyl sulfoxide (DMSO) are used during processing. Trace amounts of these ingredients may be present in LAVIV.
Fibrocell Technologies, Inc.
405 Eagleview Blvd.
Exton, PA 19341
T: 484-713-6000
F: 484-713-6001
www.fibrocellscience.com
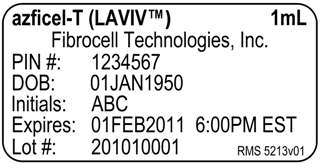
Principal Display Panel – 1mL Vial Label
azficel-T (LAVIVTM) 1mL
Fibrocell Technologies, Inc.
PIN #: 1234567
DOB: 01JAN1950
Initials: ABC
Expires: 01FEB2011 6:00PM EST
Lot #: 201010001 RMS-5213v01
Principal Display Panel – Carton Label
azficel-T
LAVIVTM
NDC# 75935-001-01 Rx Only
FOR AUTOLOGOUS USE ONLY
No preservatives. Do not freeze.
Mix gently prior to administration.
CONTENTS: Two vials each
containing approximately 18 million
human dermal fibroblasts suspended
in Dulbecco's Modified Eagle Medium
without Phenol Red.
One autologous dose for injection. See
package insert for full prescribing information
and instructions for administration.
Store Refrigerated 2-8°C
No U.S. standard of potency
NOT EVALUATED FOR INFECTIOUS SUBSTANCES
Manufactured by:
Fibrocell Technologies, Inc.
405 Eagleview Blvd., Exton, PA 19341
Telephone: (855) 365-2848
U.S. Lic. #1818 RMS-5211v01

| LAVIV
azficel-t injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Fibrocell Technologies, Inc. (067980859) |