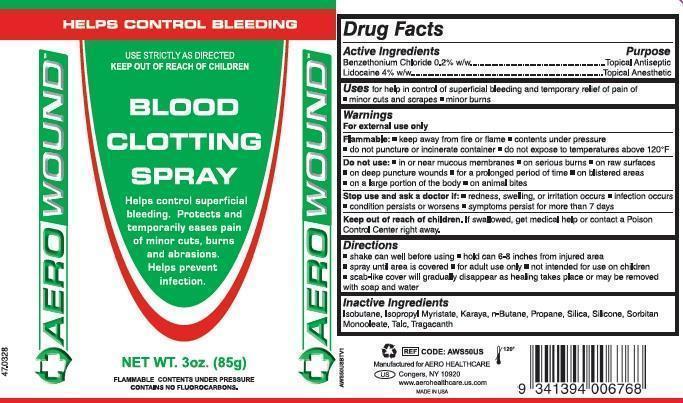
Uses
for help in control of superficial bleeding and temporary relief of pain of:
- minor cuts and scrapes
- minor burns
Warnings
For external use only
Flammable
- keep away from flame
- contents under pressure
- do not puncture or incinerate
- do not expose to temperatures above 120°F
Do not use
- in or near mucous membranes
- on serious burns
- on raw surfaces
- on deep puncture wounds
- for a prolonged period of time
- on blistered areas
- on a large portion of the body
- on animal bites
Stop use and ask doctor if
- redness, swelling, or irritation occurs
- infection occurs
- condition persists or worsens
- symptoms persist for more than 7 days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake can well before using
- hold 6-8 inches from moist injured area
- spray until area is covered
- for adult use only
- not intended for use on children
- scab-like cover will gradually disappear as healing takes place or may be removed with soap and water