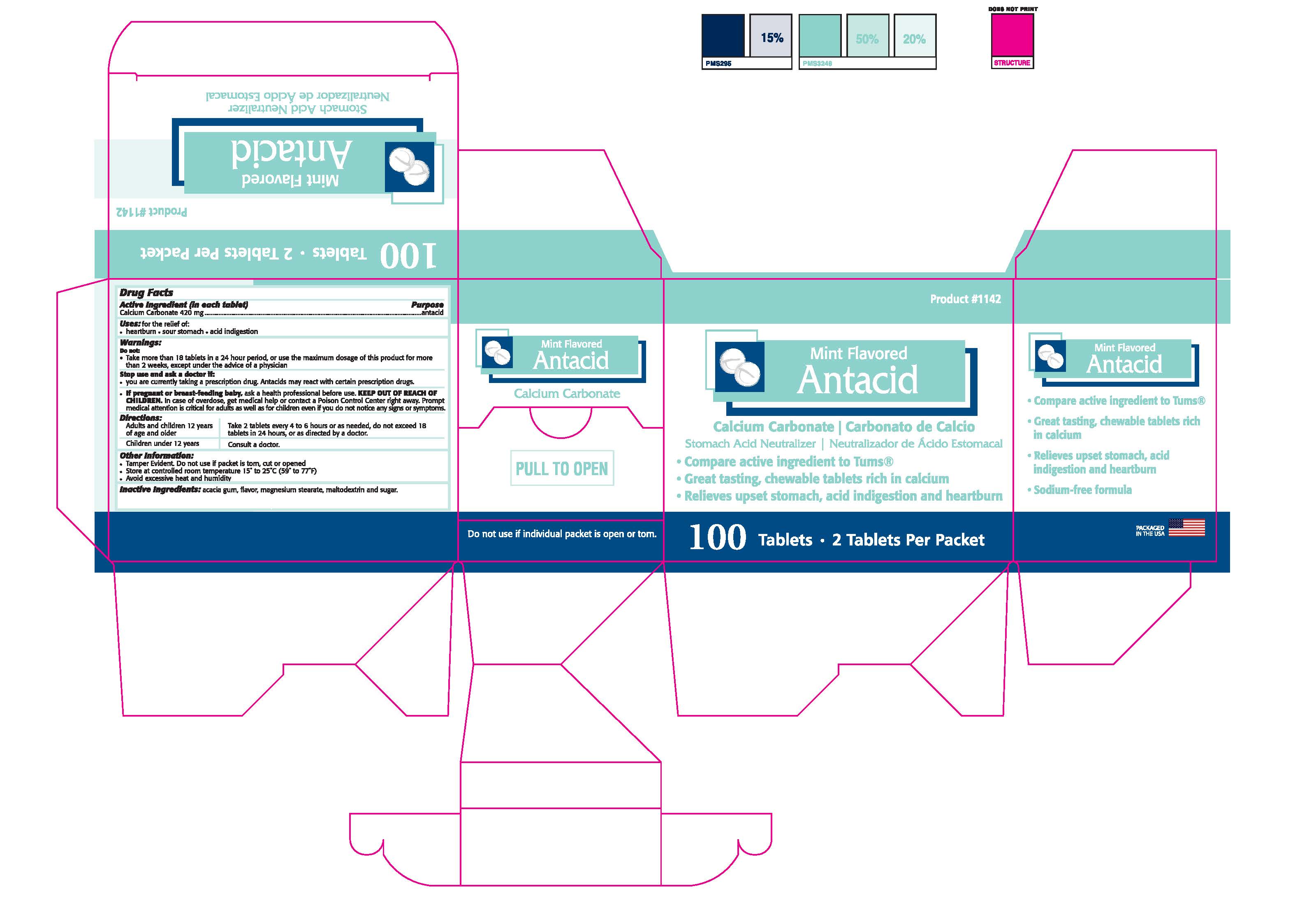
MINT ANTACID- calcium carbonate tablet, chewable
ADVANCED FIRST AID, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT IN EACH TABLET- CALCIUM CARBONATE 420 MG
Uses:
for the relief of:
•heartburn • sour stomach • acid indigestion
Warnings:
Do not:
•Take more than 18 tablets in a 24 hour period, or use the maximum dosage of
this product for more than 2 weeks, except under the advice of a physician.
Stop use and ask a doctor if:
•You are currently taking any prescription drug. Antacids may react with certain
prescription drugs.
If pregnant or breast-feeding baby, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical
attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions:
Adults and children 12 years of age and older: Take 2 tablets every 4 to
6 hours or as needed, do not exceed 18 tablets in 24 hours, or as directed by a doctor.
Children under 12 years: Consult a doctor.
Inactive Ingredients: acacia gum, flavor, magnesium stearate, maltodextrin, starch and sugar.
ADVANCED FIRST AID, INC.