DESCRIPTION
Nystatin is a polyene antifungal antibiotic drug obtained from Streptomyces nursei.
Structural formula:
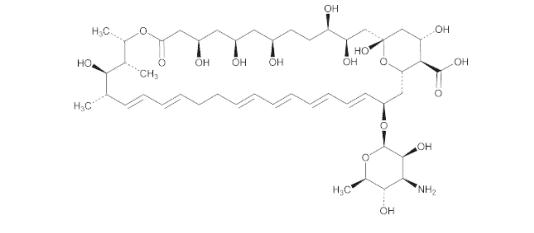
| Molecular formula
| :
| C
47H
75NO
17
|
| Molecular weight
| :
| 926.09 g/mol
|
Nystatin ointment is for dermatologic use. Nystatin ointment for topical use contains 100,000 USP nystatin units per gram in an ointment base of light mineral oil and white petrolatum.
CLINICAL PHARMACOLOGY
Microbiology
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes.
Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing concentrations of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida ( C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed.
Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
INDICATIONS AND USAGE
Nystatin ointment is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by Candida albicans and other susceptible Candida species.
Nystatin ointment is not indicated for systemic, oral, intravaginal or ophthalmic use.
CONTRAINDICATIONS
Nystatin ointment is contraindicated in patients with a history of hypersensitivity to any of their components.
PRECAUTIONS
Nystatin ointment should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
INFORMATION FOR PATIENTS
Patients using these medications should receive the following information and instructions:
1. The patient should be instructed to use these medications as directed (including the replacement of missed doses). These medications are not for any disorder other than that for which they are prescribed.
2. Even if symptomatic relief occurs within the first few days of treatment, the patient should be advised not to interrupt or discontinue therapy until the prescribed course of treatment is completed.
3. If symptoms of irritation develop, the patient should be advised to notify the physician promptly.
Laboratory Tests
If there is a lack of therapeutic response, KOH smears, cultures, or other diagnostic methods should be repeated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate the carcinogenic potential of nystatin. No studies have been performed to determine the mutagenicity of nystatin or its effects on male or female fertility.
Pregnancy: Teratogenic Effects
Category C. Animal reproduction studies have not been conducted with any nystatin topical preparation. It also is not known whether these preparations can cause fetal harm when used by a pregnant woman or can affect reproductive capacity. Nystatin topical preparations should be prescribed for a pregnant woman only if the potential benefit to the mother outweighs the potential risk to the fetus.
ADVERSE REACTIONS
The frequency of adverse events reported in patients using nystatin ointment preparations is less than 0.1%. The more common events that were reported include allergic reactions, burning, itching, rash, eczema, and pain on application.
(See PRECAUTIONS: General.)
DOSAGE AND ADMINISTRATION
Adults and Pediatric Patients (Neonates and Older):
Apply liberally to affected areas twice daily or as indicated until healing is complete.
HOW SUPPLIED
Nystatin ointment USP, 100,000 units per gram is a pale yellow to yellow color ointment available as follows:
NDC 13668-534-01 15 gram tube
NDC 13668-534-02 30 gram tube
STORAGE
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].

TORRENT PHARMACEUTICALS LTD., Pithampur-454775, INDIA .
Manufactured for:
TORRENT PHARMA INC., Basking Ridge, NJ 07920
8063926 Revised June 2018



