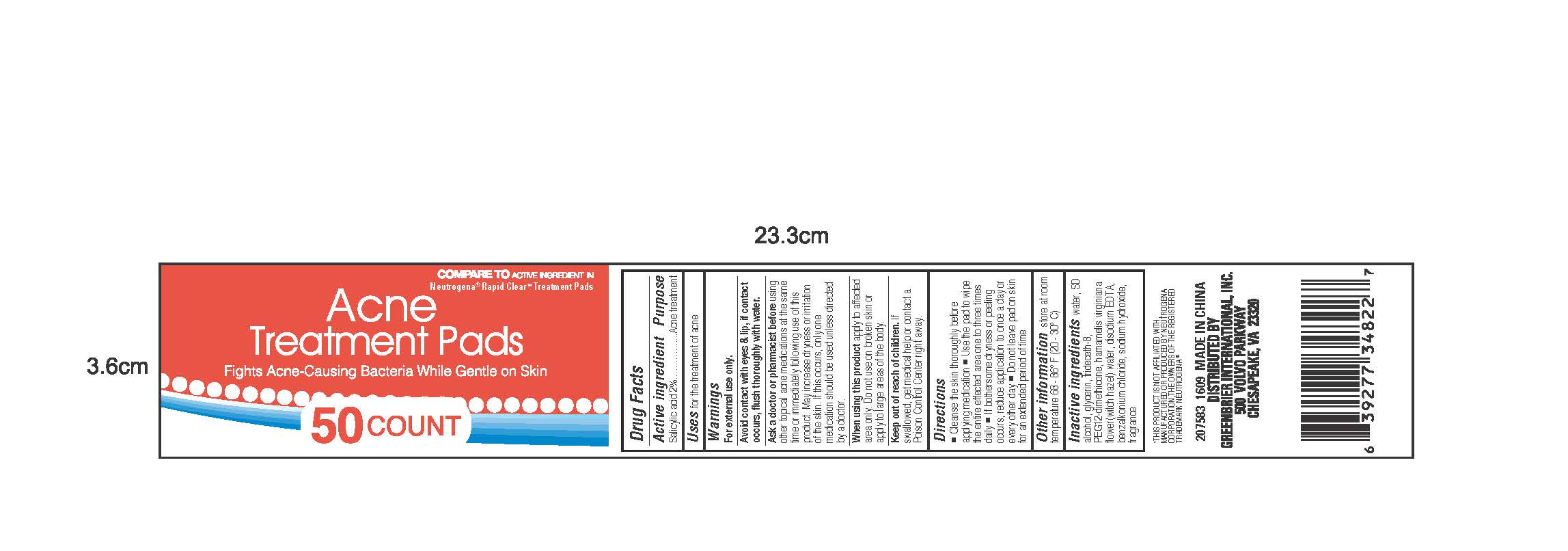
Warnings
For external use only.
Avoid contact with eyes & lip, if contact
occurs, flush throoughly with water.
Ask a doctor or pharmacist before using
other topical acne medications at the same time
or immediately following use of this product. May increase
dryness or irritation of the skin. If this occurs, only one
medication should be usedunless directed
by a doctor.
When using this product apply to affected
area only. Do not use on broken skin or
apply to large areas of the body.
Keep out of reach of children. If
swallowed, get medical help or contact a
Poison Control Center right away.
Directions
-Cleanse the skin thoroughly before applying medication
-Use the pad to wipe the entire affected area one to three times daily
-If bothersome dryness or peeling occurs, reduce application to once a day or every other day
-Do not peave pad on skin for an extended period of time.