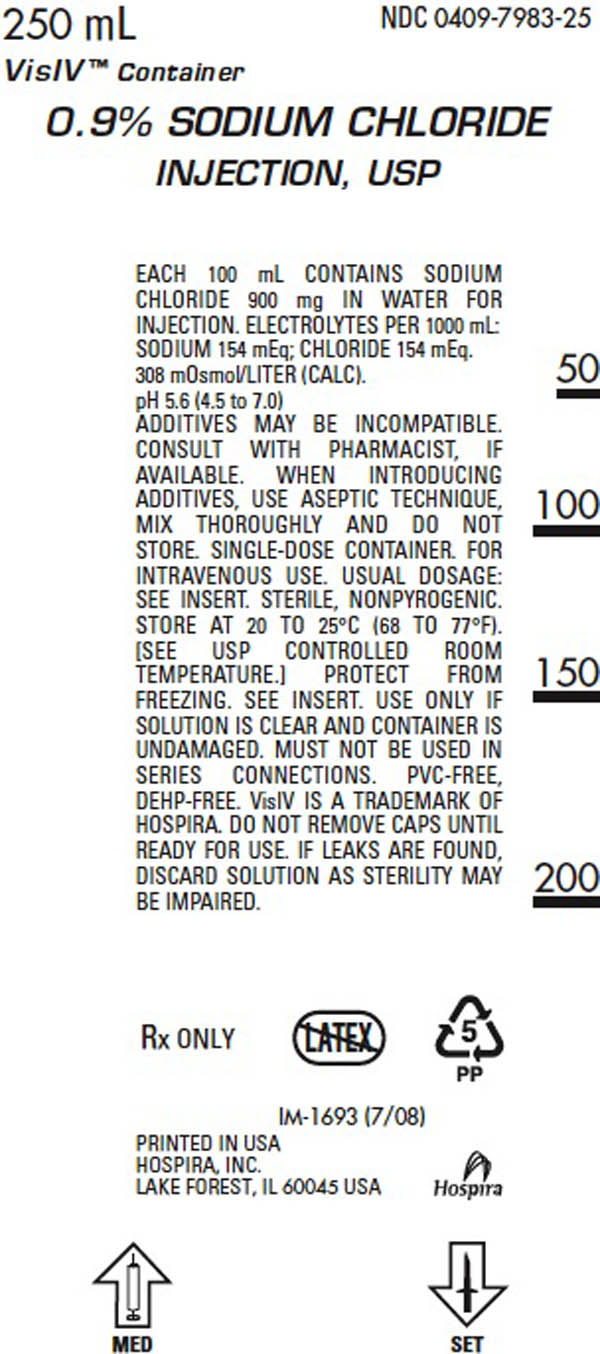
DESCRIPTION
0.9% Sodium Chloride Injection, USP is sterile and nonpyrogenic. It is a parenteral solution containing sodium chloride in water for injection intended for intravenous administration. Each 100 ml of 0.9% sodium chloride injection, USP contains 900 mg sodium chloride in water for injection. The pH is 5.6 (4.5 to 7.0). This solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only as a single-dose injection. When smaller doses are required, the unused portion should be discarded. 0.9% Sodium Chloride is a parenteral fluid and electolyte replenisher and is a white crystalline powder freely soluable in water.
 MM
MM MM
MM