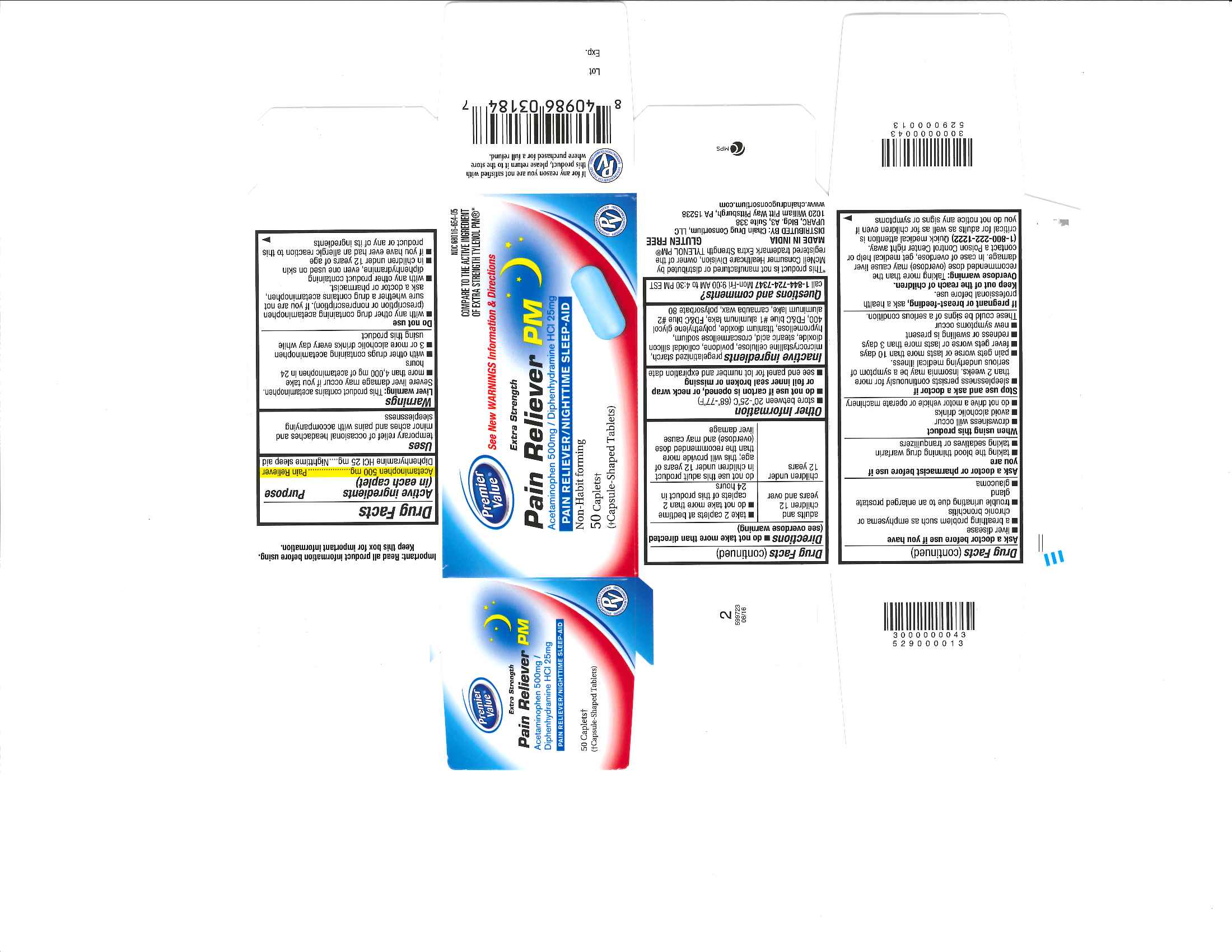
Uses
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
■ more than 4,000 mg of acetaminophen in 24 hours
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ with any other product containing diphenhydramine, even one used on skin
■ in children under 12 years of age
■ if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
■ liver disease
■ a breathing problem such as emphysema or chronic bronchitis
■ trouble urinating due to an enlarged prostate gland
■ glaucoma
Ask a doctor or pharmacist before use if you are
■ taking the blood thinning drug warfarin
■ taking sedatives or tranquilizers
When using this product
■ drowsiness will occur
■ avoid alcoholic drinks
■ do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
■ sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
■ pain gets worse or lasts more than 10 days
■ fever gets worse or lasts more than 3 days
■ redness or swelling is present
■ new symptoms occur
These could be signs of a serious condition.
Overdose warning
Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away.
(1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms
Directions
■ do not take more than directed (see overdose warning)
adults and children 12 years and over
- take 2 caplets at bedtime
- do not take more than 2 caplets of this product in 24 hours
children under 12 years
- do not use this product in children under 12 years of age; this will provide more than the recommended dose (overdose) and may cause liver damage.
Other information
■ store between 20°-25°C (68°-77°F)
■ do not use if carton is opened, or neck wrap or foil inner seal broken or mising
■ see end panel for lot number and expiration date