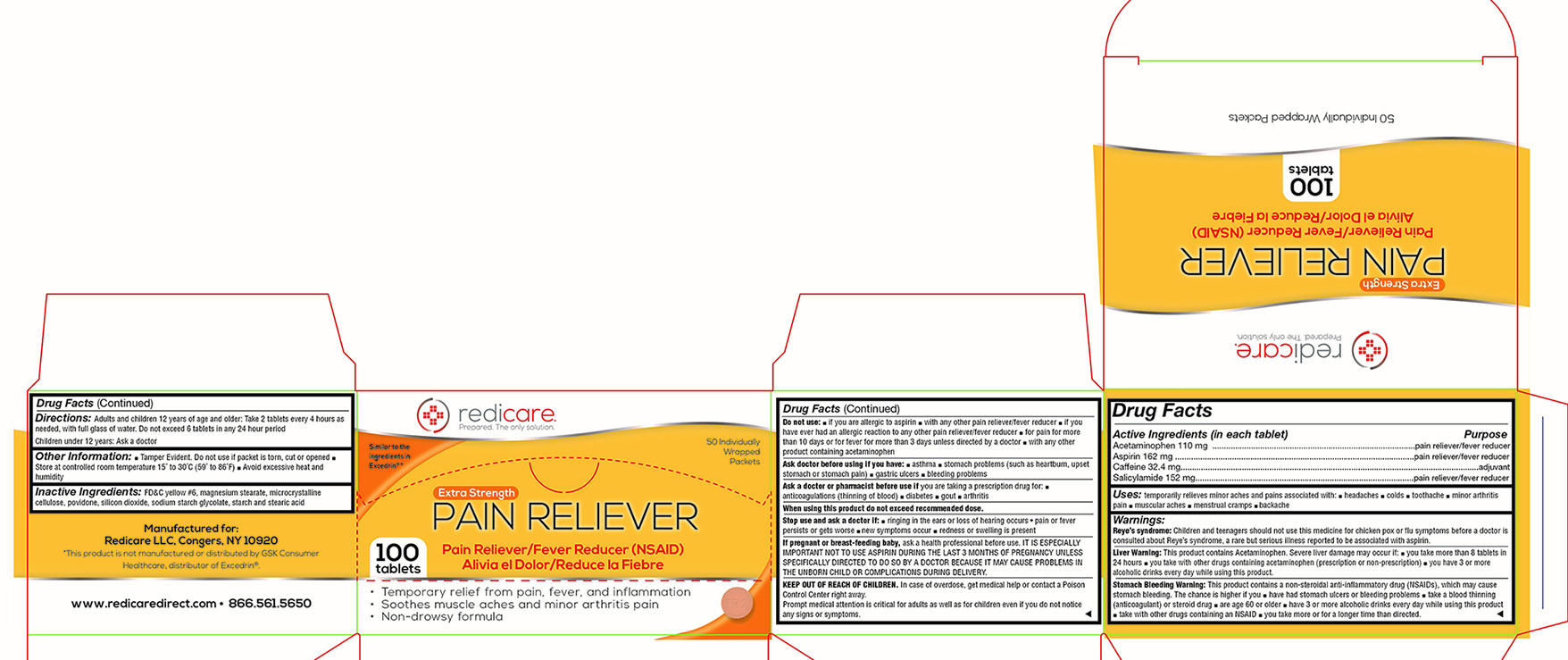
Active Ingredient
Acetaminophen 110 mg .....................................................................................pain reliever/fever reducer
Aspirin 162 mg .....................................................................................................pain reliever/fever reducer
Caffeine 32.4 mg...............................................................................................................................adjuvant
Salicylamide 152 mg............................................................................................pain reliever/fever reducer
Uses:
temporarily relieves minor aches and pains associated with ■ headaches ■ colds ■ a toothache ■ minor arthritis pain ■ muscular aches ■ menstrual cramps ■ a backache
Warnings:
Reye’s syndrome:
Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye’s syndrome, a rare but serious illness reported to be associated with aspirin.
Liver Warning:
This product contains Acetaminophen. Severe liver damage may occur if: ■ you take more than 8 tablets in 24 hours ■ you take with other drugs containing acetaminophen (prescription or non-prescription) ■ you have 3 or more alcoholic drinks every day while using this product.
Stomach Bleeding Warning:
This product contains a non-steroidal anti-inflammatory drug (NSAIDs), which may cause stomach bleeding. The chance is higher if you ■ have had stomach ulcers or bleeding problems ■ take a blood thinning (anticoagulant) or steroid drug ■ are age 60 or older ■ have 3 or more alcoholic drinks every day while using this product ■ take with other drugs containing a NSAID ■ you take more or for a longer time than directed.
Do not use:
■ if you are allergic to aspirin ■ with any other pain reliever/fever reducer ■ if you have ever had an allergic reaction to any other pain reliever/fever reducer ■ for pain for more than 10 days or for fever for more than 3 days unless directed by a doctor ■ with any other product containing acetaminophen
Ask doctor before using if you have:
■ asthma ■ stomach problems (such as heartburn, upset stomach or stomach pain) ■ gastric ulcers ■ bleeding problems
Ask a doctor or pharmacist before use if
you are taking a prescription drug for ■ anticoagulation (thinning of blood) ■ diabetes ■ gout ■ arthritis
Stop use and ask a doctor if
■ ringing in the ears or loss of hearing occurs • pain or fever persists or gets worse ■ new symptoms occur ■ redness or swelling is present
If pregnant or breast-feeding a baby,
ask a health professional before use. IT IS ESPECIALLY IMPORTANT NOT TO USE ASPIRIN DURING THE LAST 3 MONTHS OF PREGNANCY UNLESS SPECIFICALLY DIRECTED TO DO SO BY A DOCTOR BECAUSE IT MAY CAUSE PROBLEMS IN THE UNBORN CHILD OR COMPLICATIONS DURING DELIVERY.
KEEP OUT OF REACH OF CHILDREN.
In a case of overdose, get medical help or contact a Poison Control Center right away.
Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions:
Adults and children 12 years of age and older: Take 2 tablets every 4 hours as needed, with a full glass of water. Do not exceed 6 tablets in any 24 hour period
Children under 12 years: Ask a doctor
Other Information:
■ Tamper Evident. Do not use if packet is torn, cut or opened ■ Store at controlled room temperature 15˚ to 30˚C (59˚ to 86˚F) ■ Avoid excessive heat and humidity