GENERAL:
This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
DESCRIPTION:
This product is a keratolytic, emollient which is a gentle, yet potent, tissue softener for skin.
Each gram contains 390 mg of urea in a vehicle consisting of: carbomer, cetyl alcohol, dimethyl isosorbide, glyceryl stearate, mineral oil, petrolatum, propylene glycol, sodium hydroxide, water, and xanthan gum.
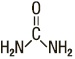
Urea is a diamide of carbonic acid with the following chemical structure:
CLINICAL PHARMACOLOGY:
Urea gently dissolves
the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas of the skin.
INDICATIONS:
This product is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
CONTRAINDICATIONS:
This product is contraindicated in persons with known or suspected hypersensitivity to any of the ingredients of the product.
ADVERSE REACTIONS:
Transient stinging, burning, itching or irritation may occur and normally disappear upon discontinuing the use of this product.
DOSAGE AND ADMINISTRATION:
Apply to affected area(s) twice per day or as directed by a physician. Rub in until completely absorbed.
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (between 59°F to 86°F). Brief exposure to
temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
INFORMATION FOR PATIENTS:
Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or
elsewhere. Avoid contact with eyes, lips and mucous membranes.
CARCINOGENISIS, MUTAGENESIS AND IMPAIRMENT OF FERTILITY:
Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed.
Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
