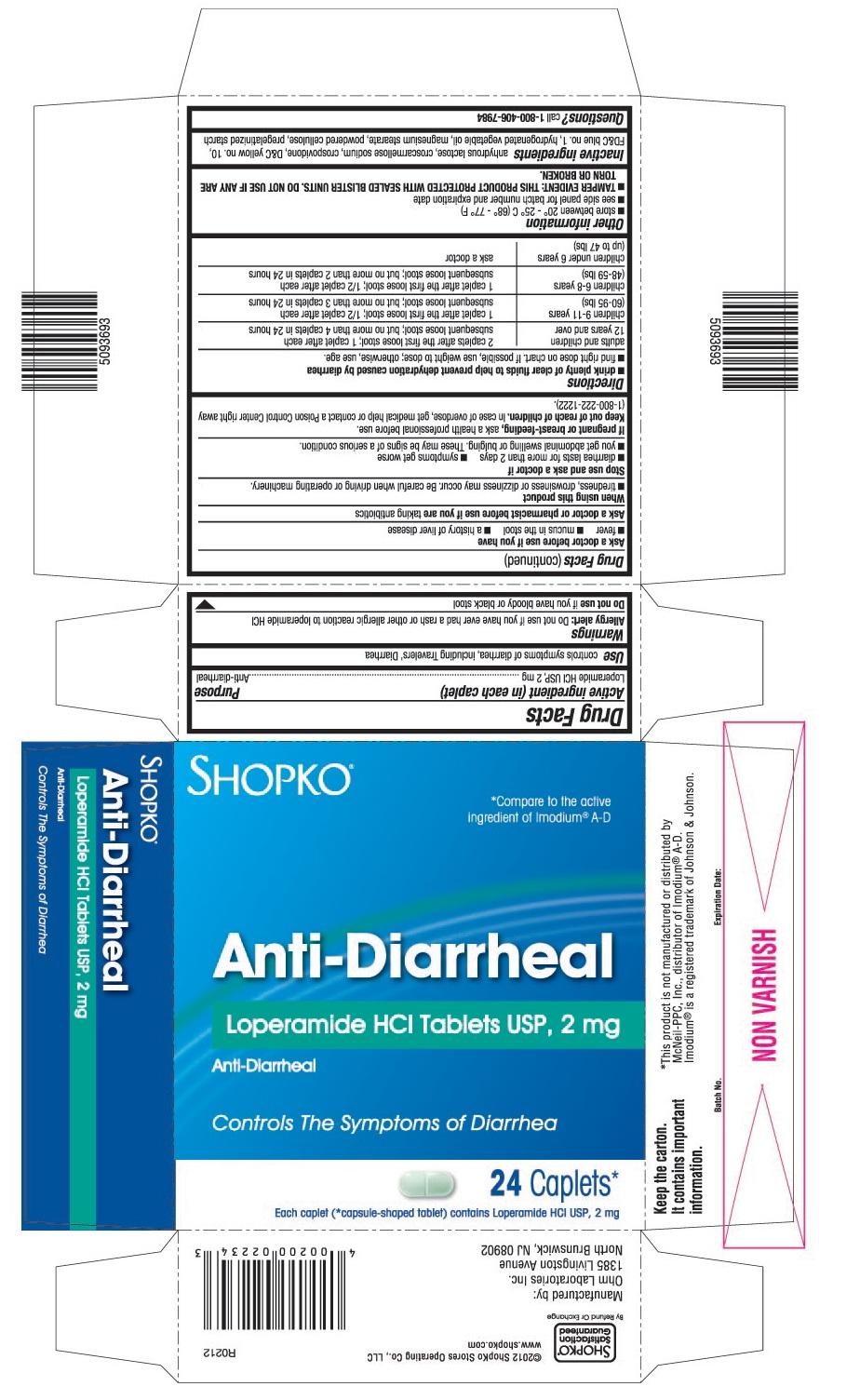
WARNINGS
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCI
When using this product
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
DIRECTIONS
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool;
1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hourschildren 9-11 years
(60-95 lbs)1 caplet after the first loose stool;
½ caplet after each subsequent loose stool; but no more than 3 caplets in 24 hourschildren 6-8 years
(48-59 lbs)1 caplet after the first loose stool;
½ caplet after each subsequent loose stool; but no more than 2 caplets in 24 hourschildren under 6 years
(up to 47 lbs)ask a doctor
OTHER INFORMATION
- store between 20° – 25° C (68° – 77° F)
- see side panel for batch number and expiration date
- TAMPER EVIDENT: THIS PRODUCT PROTECTED WITH SEALED BLISTER UNITS. DO NOT USE IF ANY ARE TORN OR BROKEN.
INACTIVE INGREDIENTS
Anhydrous lactose, croscarmellose sodium, crospovidone, D&C yellow no.10, FD&C blue no.1, hydrogenated vegetable oil, magnesium stearate, powdered cellulose, pregelatinized starch