FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is an osmotic laxative indicated for cleansing of the colon as a preparation for colonoscopy in adults.
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Correct fluid and electrolyte abnormalities before treatment with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Warnings and Precautions (5.1)].
- Two doses of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution are required for a complete preparation for colonoscopy. The time interval between the two doses depends on the regimen prescribed and the planned timing of the colonoscopy procedure. [see Dosage and Administration (2.2, 2.3 )] .
- The "Split-Dose" is the preferred method and consists of two separate doses: the first dose is taken the evening before the colonoscopy, and the second dose is taken the next day, the morning of the day of the colonoscopy [see Dosage and Administration (2.2)].
- The "Evening Only" is the alternative method and consists of two separate doses: both doses are taken in the evening before the day of the colonoscopy, with a minimum of 1.5 hours between the start of the first dose and the start of the second dose [see Dosage and Administration (2.3)].
- Both PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution dosing regimens require administration of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution using the mixing container provided to reconstitute the contents of Pouch A, Pouch B and Lemon flavor pack with water to the Fill Line.
- Do not reconstitute with other liquids and/or add starch-based thickeners to the mixing container [see Warnings and Precautions (5.7)].
- Additional clear liquids (including water) must be consumed in both dosing regimens [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)] .
- Consume only clear liquids (no solid food) from the start of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution treatment until after the colonoscopy.
- Do not eat or drink alcohol, milk, anything colored red or purple or any other foods containing pulp material.
- Do not take other laxatives while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Drug Interactions (7.3)] .
- Do not take oral medications within 1 hour before or after starting each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Drug Interactions (7.2)].
- Ensure completion of Dose 2, including all additional liquids, at least 2 hours before the colonoscopy.
- Storage : After reconstitution, store PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution- solution in an upright position and keep refrigerated. Use within 24 hours after it is mixed in water.
2.2 Two-Day Split-Dosing Regimen (Preferred Method)
The Two-Day Split-Dosing regimen is the preferred dosing method.
Instruct adult patients that on the day before the clinical procedure, they can consume breakfast, followed by a light lunch (no solid foods), and clear soup and/or plain yogurt for dinner, which must be completed at least 1 hour prior to the start of the first PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution dose.
Instruct adult patients to take two separate doses in conjunction with fluids as follows:
Dose 1 – In the evening before the colonoscopy, approximately 10 to 12 hours before Dose 2:
- Empty the contents of 1 Pouch A, 1 Pouch B and 1 Lemon Flavor pack into the mixing container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- Add lukewarm water to the Fill Line on the mixing container (32 fluid ounces). Do not add other ingredients to the PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution- solution.
- Thoroughly mix with a spoon or shake with lid on securely until the contents of Pouch A, Pouch B and Lemon flavor pack are completely dissolved.
- Drink 8 ounces of the solution every 15 minutes. This should take about 1 hour. Be sure to drink all the solution.
- Refill the mixing container halfway to the Fill Line (at least 16 ounces) with a clear liquid and drink all this liquid before going to bed.
Dose 2 – Take next morning, on the day of the colonoscopy, approximately 12 hours after the start of Dose 1 and at least 3 ½ hours prior to colonoscopy:
- Empty the contents of 1 Pouch A, 1 Pouch B and 1 Lemon flavor pack into the mixing container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- Add lukewarm water to the Fill Line on the mixing container (32 fluid ounces). Do not add other ingredients to the PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution- solution.
- Thoroughly mix with a spoon or shake with lid on securely until the contents of Pouch A, Pouch B and Lemon flavor pack are completely dissolved.
- Drink 8 ounces of the solution every 15 minutes. This should take about 1 hour. Be sure to drink all of the solution.
- Refill the mixing container halfway to the Fill Line (at least 16 ounces) with a clear liquid and drink all this liquid at least 2 hours before the colonoscopy.
- Consume additional water or clear liquids up to 2 hours before the colonoscopy or as prescribed by your healthcare provider. Then stop drinking liquids until after the colonoscopy.
Stop drinking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution temporarily or drink each portion at longer intervals if severe bloating, abdominal discomfort or distention occurs, until these symptoms resolve.
2.3 One-Day Evening Only Dosing Regimen (Alternative Method)
The One-Day Evening Only regimen is the alternative dosing method for patients for whom the Split-Dosing regimen is inappropriate.
Instruct adult patients that on the day before the clinical procedure, they can consume breakfast, followed by a light lunch (no solid foods), and clear soup and/or plain yogurt for dinner, which must be completed at least 1 hour prior to the start of the first PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution dose.
Instruct adult patients to take two separate doses in conjunction with fluids as follows:
Dose 1 – At least 3 ½ hours before bedtime the evening before the colonoscopy:
- Empty the contents of 1 Pouch A, 1 Pouch B and 1 Lemon Flavor pack into the mixing container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- Add lukewarm water to the Fill Line on the mixing container (32 fluid ounces). Do not add other ingredients to the PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution- solution.
- Thoroughly mix with a spoon or shake with lid on securely until the contents of Pouch A, Pouch B and Lemon Flavor pack are completely dissolved.
- Drink 8 ounces of the solution every 15 minutes. This should take about 1 hour. Be sure to drink all the solution.
Dose 2 – At least 1 ½ hours after starting Dose 1 on the evening before the colonoscopy:
- Empty the contents of 1 Pouch A, 1 Pouch B and 1 Lemon Flavor pack into the mixing container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- Add lukewarm water to the Fill Line on the mixing container (32 fluid ounces). Do not add other ingredients to the PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution- solution.
- Thoroughly mix with a spoon or shake with lid on securely until the contents of Pouch A, Pouch B and Lemon Flavor pack are completely dissolved.
- Drink 8 ounces of the solution every 15 minutes. This should take about 1 hour. Be sure to drink all of the solution.
- Refill the mixing container to the Fill Line (32 fluid ounces) with a clear liquid and drink all this liquid before going to bed.
- Consume additional water or clear liquids up to 2 hours before the colonoscopy or as prescribed by your healthcare provider. Then stop drinking liquids until after the colonoscopy.
Stop drinking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution temporarily or drink each portion at longer intervals if severe bloating, abdominal discomfort or distention occurs, until these symptoms resolve.
3 DOSAGE FORMS AND STRENGTHS
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is supplied as a white to yellow free flowing powder with odor of lemon for reconstitution and is available in a carton that contains 2 pouches labeled Pouch A, 2 pouches labeled Pouch B and 2 Pouches labeled as Lemon Flavor pack.
- Each Pouch A contains 100 grams of polyethylene glycol (PEG) 3350, NF, 7.5 grams of sodium sulfate, USP, 2.691 grams of sodium chloride, USP, and 1.015 grams of potassium chloride, USP.
- Each Pouch B contains 4.7 grams of ascorbic acid, USP and 5.9 grams of sodium ascorbate, USP.
- Each Lemon Flavor pack contains natural lemon flavor, maltodextrin and saccharin sodium.
4 CONTRAINDICATIONS
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is contraindicated in the following conditions:
- Gastrointestinal (GI) obstruction [see Warnings and Precautions (5.6)]
- Bowel perforation [see Warnings and Precautions (5.6)]
- Gastric retention
- Ileus
- Toxic colitis or toxic megacolon
- Hypersensitivity to any ingredient in PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Warnings and Precautions (5.10)]
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Electrolyte Abnormalities
Advise patients to hydrate adequately before, during, and after the use of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. If a patient develops significant vomiting or signs of dehydration after taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN).
Bowel preparations can cause fluid and electrolyte disturbances, which can lead to serious adverse reactions including cardiac arrhythmias, seizures, and renal impairment [see Adverse Reactions (6.2)]. Correct fluid and electrolyte abnormalities before treatment with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution should be used with caution in patients using concomitant medications that increase the risk of electrolyte abnormalities [such as diuretics, angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs)] or in patients with known or suspected hyponatremia. Consider performing pre-dose and post-colonoscopy laboratory tests (sodium, potassium, calcium, creatinine, and BUN) in these patients [see Drug Interactions (7.1)].
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias (including atrial fibrillation) associated with the use of ionic osmotic laxative products for bowel preparation. These occur predominantly in patients with underlying cardiac risk factors and electrolyte disturbances. Use caution when prescribing PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, cardiomyopathy, or electrolyte imbalance). Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been rare reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia [see Drug Interactions (7.1)].
5.4 Use in Patients with Renal Impairment
Use PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with caution in patients with renal impairment or patients taking concomitant medications that affect renal function (such as diuretics, ACE inhibitors, angiotensin receptor blockers, or nonsteroidal anti-inflammatory drugs) [see Drug Interactions (7.1)]. These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration before, during, and after use of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, and consider performing pre-dose and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Use in Specific Populations (8.6)].
5.5 Colonic Mucosal Ulceration, Ischemic Colitis and Ulcerative Colitis
Osmotic laxatives may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution may increase the risk of mucosal ulceration or ischemic colitis and is not recommended. Consider the potential for mucosal ulcerations resulting from the bowel preparation when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease.
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Contraindications (4)].
Use with caution in patients with severe ulcerative colitis.
5.7 Aspiration
Patients with impaired gag reflex or other swallowing abnormalities are at risk for regurgitation or aspiration of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. Observe these patients during the administration of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. Use with caution in these patients.
Do not combine PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with starch-based thickeners [see Dosage and Administration (2.1)]. Polyethylene glycol (PEG), a component of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, when mixed with starch-thickened liquids reduces the viscosity of the starch-thickened liquid. When a PEG-based product used for another indication was mixed in starch-based pre-thickened liquids used in patients with dysphagia, thinning of the liquid occurred and cases of choking and potential aspiration were reported.
5.8 Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency
Since PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution contains sodium ascorbate and ascorbic acid, PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution should be used with caution in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, especially G6PD deficiency patients with an active infection, with a history of hemolysis, or taking concomitant medications known to precipitate hemolytic reactions.
5.10 Hypersensitivity Reactions
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution contains polyethylene glycol (PEG) and lemon flavoring (containing Natural Lemon Flavor, Maltodextrin and Saccharin Sodium) and may cause serious hypersensitivity reactions including anaphylaxis, angioedema, rash, urticaria, and pruritus [see Contraindications (4), Adverse Reactions (6.2) and Description (11)]. Inform patients of the signs and symptoms of anaphylaxis, and instruct them to seek immediate medical care should signs and symptoms occur.
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling:
- Serious Fluid and Electrolyte Abnormalities [see Warnings and Precautions (5.1)]
- Cardiac Arrhythmias [see Warnings and Precautions (5.2)]
- Seizures [see Warnings and Precautions (5.3)]
- Patients with Renal Impairment [see Warnings and Precautions (5.4)]
- Colonic Mucosal Ulceration, Ischemic Colitis and Ulcerative Colitis [see Warnings and Precautions (5.5)]
- Patients with Significant Gastrointestinal Disease [see Warnings and Precautions (5.6)]
- Aspiration [see Warnings and Precautions (5.7)]
- Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency [see Warnings and Precautions (5.8)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.10)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution as a Two-Day Split-Dosing and One-Day Evening Only Dosing Regimen was evaluated in two randomized, active-controlled, multicenter, investigator-blinded clinical trials in adult patients scheduled to have an elective colonoscopy [see Clinical Studies (14)]. The safety analysis for Study 1 included 359 adult patients ranging in age from 18 to 88 years (mean age 59), with 52% female and 48% male patients. The safety analysis for Study 2 included 340 adult patients ranging in age from 21 to 76 years (mean age 53), with 53% male and 47% female patients.
Tables 1 and 2 display adverse reactions reported in at least 2% and 5% of patients in either treatment group in Study 1 and Study 2, respectively. Since diarrhea was considered as a part of the efficacy assessment, it was not defined as an adverse reaction in these trials.
| PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution
Two-Day Split Dosing Regimen (N=180) |
4 Liter PEG + Electrolytes Solution (N=179) |
|
| Malaise | 19% | 18% |
| Nausea | 14% | 20% |
| Abdominal pain | 13% | 15% |
| Vomiting | 8% | 13% |
| Upper abdominal pain | 6% | 6% |
| Dyspepsia | 3% | 1% |
| 1 Reported in at least 2% of patients in either treatment group |
||
| PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution
One-Day Evening Only Dosing Regimen(N=169) |
90 mL Oral Sodium Phosphate Solution (N=171) |
|
| Abdominal distension | 60% | 41% |
| Anal discomfort | 51% | 52% |
| Thirst | 47% | 65% |
| Nausea | 47% | 47% |
| Abdominal pain | 39% | 32% |
| Sleep disorder | 35% | 29% |
| Rigors | 34% | 30% |
| Hunger | 30% | 71% |
| Malaise | 27% | 53% |
| Vomiting | 7% | 8% |
| Dizziness | 7% | 18% |
| Headache | 2% | 5% |
| Hypokalemia | 0% | 6% |
| Hyperphosphatemia | 0% | 6% |
| 1Reported in at least 5% of patients in either treatment group 2Patients were specifically asked about the occurrence of the following symptoms: shivering, anal irritations, abdominal bloating or fullness, sleep loss, nausea, vomiting, weakness, hunger sensation, abdominal cramps or pain, thirst sensation, and dizziness. |
||
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution or other PEG-based products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: Tachycardia, palpitations, hypertension, arrhythmia, atrial fibrillation, peripheral edema, asystole, acute pulmonary edema and syncope, and dehydration.
Gastrointestinal: upper gastrointestinal bleeding from a Mallory-Weiss tear, esophageal perforation [usually with gastroesophageal reflux disease (GERD)]
Hypersensitivity reactions: anaphylaxis (some of which were severe, including shock), rash, urticaria, pruritus, lip, tongue and facial swelling, dyspnea, chest tightness and throat tightness, rhinorrhea, dermatitis, fever, and chills [see Warnings and Precautions (5.10)].
Nervous system: tremor, seizure.
Renal: renal impairment and/or failure.
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Risks due to Fluid and Electrolyte Abnormalities
Use caution when prescribing PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for patients with conditions and/or who are using medications that increase the risk for fluid and electrolyte disturbances or may increase the risk of renal impairment, seizures, arrhythmias, or QT prolongation in the setting of fluid and electrolyte abnormalities [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)].
Consider additional patient evaluations as appropriate
7.2 Potential for Reduced Drug Absorption
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution can reduce the absorption of other co-administered drugs. Administer oral medications at least 1 hour before the start of administration of each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Dosage and Administration (2.1)].
7.3 Stimulant Laxatives
Concurrent use of stimulant laxatives and PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution may increase the risk of mucosal ulceration or ischemic colitis. Avoid use of stimulant laxatives (e.g., bisacodyl, sodium picosulfate) while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution [see Warnings and Precautions (5.5, 5.6)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no available data on PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Animal reproduction studies have not been conducted with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
There are no data available on the presence of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution in human milk, the effects of the drug on the breastfed child, or the effects of the drug on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution to a child during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution and any potential adverse effects on the breastfed child from PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution in pediatric patients have not been established.
8.5 Geriatric Use
Of the 413 patients in clinical trials receiving PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, 91 (22%) patients were aged 65 or older, while 25 (6%) patients were over 75 years of age. No overall differences in safety or effectiveness were observed between geriatric patients and younger patients, and other reported clinical experience has not identified differences in responses between geriatric patients and younger patients. However, elderly patients are more likely to have decreased hepatic, renal or cardiac function and may be more susceptible to adverse reactions resulting from fluid and electrolyte abnormalities [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Use PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with caution in patients with renal impairment or patients taking concomitant medications that may affect renal function [see Drug Interactions (7.1)]. These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration before, during and after the use of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Warnings and Precautions (5.4)].
10 OVERDOSAGE
Overdosage of more than the recommended dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution may lead to severe electrolyte disturbances, including hyponatremia and/or hypokalemia, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances. Certain severe electrolyte disturbances may lead to cardiac arrhythmias, seizures, and renal failure [see Warnings and Precautions (5.1, 5.2, 5.3)]. Monitor for fluid and electrolyte disturbances and treat symptomatically.
11 DESCRIPTION
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is an osmotic laxative consisting of 6 pouches (2 of Pouch A, 2 of Pouch B and 2 of Lemon Flavor Pack) containing white to yellow free flowing powder with odor of lemon for reconstitution.
Each Pouch A contains 100 grams of polyethylene glycol (PEG) 3350, NF, 7.5 grams of sodium sulfate, USP, 2.691 grams of sodium chloride, USP, and 1.015 grams of potassium chloride, USP. Pouch A contains 111.2 g of powder for oral solution.
Each Pouch B contains 4.7 grams of ascorbic acid, USP and 5.9 grams of sodium ascorbate, USP. Pouch B contains 10.6 g of powder for oral solution.
Each Lemon Flavor pack contains natural lemon flavor; maltodextrin and saccharin sodium.
When 1 Pouch A, 1 Pouch B and 1 Lemon Flavor pack are dissolved together in water to a volume of 1 liter, PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is an oral solution having a lemon taste. The entire, reconstituted, 2-liter PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution colon preparation contains 200 grams of PEG-3350, 15 grams of sodium sulfate, 5.38 grams of sodium chloride, 2.03 grams of potassium chloride, 9.4 grams of ascorbic acid, and 11.8 grams of sodium ascorbate plus the following excipients: sodium saccharin, USP, maltodextrin, NF, and lemon flavoring.
A mixing container for reconstitution is enclosed.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary mode of action is osmotic action of polyethylene glycol 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid, which induce a laxative effect. The physiological consequence is increased water retention in the lumen of the colon, resulting in loose stools.
14 CLINICAL STUDIES
The colon cleansing efficacy and safety of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution was evaluated in two randomized, actively-controlled, multi-center, investigator-blinded trials in adult patients scheduled to have an elective colonoscopy.
In Study 1, patients were randomized to one of the following two colon preparation treatments: 1) 2 liters of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with 1 additional liter of clear liquid split into two doses (during the evening before and the morning of the colonoscopy) and 2) 4 liters of polyethylene glycol plus electrolytes solution (4L PEG + E) split into two doses (during the evening before and the morning of the colonoscopy). Patients were allowed to have a morning breakfast, a light lunch, clear soup and/or plain yogurt for dinner. Dinner had to be completed at least one hour prior to initiation of the colon preparation administration.
The primary efficacy endpoint was the proportion of patients with effective colon cleansing as judged by blinded gastroenterologists on the basis of videotapes recorded during the colonoscopy.
The blinded gastroenterologists graded the colon cleansing twice (during introduction and withdrawal of the colonoscope) and the poorer of the two assessments was used in the primary efficacy analysis.
The efficacy analysis included 308 adult patients who had an elective colonoscopy. Patients ranged in age from 18 to 88 years old (mean age about 59 years old) with 52% female and 48% male patients. Table 3 displays the results.
| Responders A 2 or B 3 (%)
| C 4 (%)
| D 5 (%)
|
|
| PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution (N=153)
| 88.9 | 9.8 | 1.3 |
| 4L PEG + E 1 (N=155)
| 94.8 | 4.5 | 0.6 |
| 14L PEG + E is 4 Liter Polyethylene Glycol plus Electrolytes Solution. 2A: colon empty and clean or presence of clear liquid, but easily removed by suction 3B: brown liquid or semisolid remaining amounts of stool, fully removable by suction or displaceable, thus allowing a complete visualization of the gut mucosa 4C: semisolid amounts of stool, only partially removable with a risk of incomplete visualization of the gut mucosa 5D: semisolid or solid amounts of stool; consequently colonoscopy incomplete or needed to be terminated. |
|||
4L PEG+E's responder rate was not significantly higher than PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution's responder rate.
In Study 2, patients were randomized to one of the following two colon preparation treatments: 1) 2 liters of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with 1 additional liter of clear liquid in the evening prior to the colonoscopy and 2) 90 mL of oral sodium phosphate solution (90 mL OSPS) with at least 2 liters of additional clear liquid during the day and evening prior to the colonoscopy. Patients randomized to PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution therapy were allowed to have a morning breakfast; a light lunch; and clear soup and/or plain yogurt for dinner. Dinner had to be completed at least one hour prior to initiation of the colon preparation administration.
The primary efficacy endpoint was the proportion of patients with effective colon cleansing as judged by the colonoscopist and one blinded gastroenterologist (on the basis of videotapes recorded during the colonoscopy). In case of a discrepancy between the colonoscopist and the blinded gastroenterologist, a second blinded gastroenterologist made the final efficacy determination.
The efficacy analysis included 280 adult patients who had an elective colonoscopy. Patients ranged in age from 21 to 76 years old (mean age about 53 years old) with 47% female and 53% male patients. Table 4 displays the results.
| Responders A 2 or B 3 (%)
| C 4 (%)
| D 5 (%)
|
|
| PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution (N=137)
| 73.0 | 23.4 | 3.6 |
| 90 mL OSPS 1 (N=143)
| 64.4 | 29.4 | 6.3 |
| 1OSPS is Oral Sodium Phosphate Solution. 2A: empty and clean or clear liquid (transparent, yellow, or green) 3B: brown liquid or semisolid remaining small amounts of stool, fully removable by suction or displaceable allowing a complete visualization of the underlying mucosa 4C: semisolid only partially removable/displaceable stools; risk of incomplete examination of the underlying mucosa 5D: heavy and hard stool making the segment examination uninterpretable and, consequently, the colonoscopy needed to be terminated. |
|||
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution's responder rate was not significantly higher than OSPS's responder rate.
16 HOW SUPPLIED/STORAGE AND HANDLING
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is supplied as a white to yellow free flowing powder with odor of lemon for reconstitution.
Pouch A contains 100 grams of PEG 3350, NF; 7.5 grams of sodium sulfate, NF; 2.691 grams of sodium chloride, USP/NF; and 1.015 gram of potassium chloride, USP/NF: NDC 0093-9044-19.
Pouch B contains 4.7 grams of Ascorbic acid; 5.9 grams of sodium ascorbate, USP/NF: NDC 0093-9043-19.
Lemon flavor pack contains natural lemon flavor; maltodextrin and saccharin sodium.
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, mixing container: NDC 0093-9045-19
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, single-use inner carton: The inner carton contains three pouches labeled Pouch A, Pouch B and lemon flavor pack: NDC 0093-3560-19.
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, single-use outer carton: Each outer carton contains 2 inner cartons, prescribing information and patient information and a mixing container with lid for reconstitution of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution: NDC 0093-3560-26
Storage
Store carton/container at room temperature, between 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).When reconstituted, store upright and keep solution refrigerated. Use within 24 hours [see Dosage and Administration (2.1)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Instruct patients:
- That two doses of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution are required for a complete preparation for colonoscopy either as a Split-Dose (2-Day), or Evening Only (1-Day) dosing regimen [see Instructions for Use] .
- Not to take other laxatives while they are taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- That each pouch needs to be reconstituted in water before ingestion and that they should drink additional clear liquids. Examples of clear liquids can be found in the Instructions for Use .
- Not to take oral medications within one hour of starting each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- To follow the directions in the Instructions for Use , for either the Two-Day Split-Dosing or the One-Day Evening Only Dosing regimen, as prescribed.
- To consume additional clear liquids before, during, and after the use of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution to prevent dehydration [see Warnings and Precautions (5.1)] .
- To contact their healthcare provider if they develop significant vomiting or signs of dehydration after taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution or if they experience altered consciousness or seizures [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)]
- Not to eat or drink alcohol, milk, anything colored red or purple or any other foods containing pulp material.
- To stop drinking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution temporarily or drink each portion at longer intervals if they develop severe abdominal discomfort or distention until these symptoms diminish. If severe symptoms persist, tell patients to contact their healthcare provider.
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
SAP Code: 270438
Rev. 04/2022
MEDICATION GUIDE
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution
(pol˝ ee eth´ i leen glye´ kol, soeˊ dee um sul' fate, soeˊ dee um klor´ ide, poe tasˊee um klor´ ide, soeˊ dee um a skor´ bate, as koreˊ bik as´ id for oral solution)
Read this Medication Guide and Instructions for Use before your colonoscopy and again before you start taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
What is the most important information I should know about PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution? PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution and other bowel preparations can cause serious side effects, including:
- Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood. These changes can cause:
- abnormal heartbeats that can cause death.
- seizures. This can happen even if you have never had a seizure.
- kidney problems.
Your chance of having fluid loss and changes in body salts with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is higher if you:
- have heart problems.
- have kidney problems.
- take water pills (diuretics), high blood pressure medicine, or non-steroidal anti-inflammatory drugs (NSAIDS).
Tell your healthcare provider right away if you have any of these symptoms of serious loss of body fluid (dehydration) while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution:
- vomiting
- dizziness
- urinating less often than normal
- headache
See "What are the possible side effects of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?" for more information about side effects.
What is PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is a prescription medicine used by adults to clean the colon before a colonoscopy. PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution cleans your colon by causing you to have diarrhea (loose stools). Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy.
It is not known if PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution is safe and effective in children.
Who should not take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?
Do not take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution if your healthcare provider has told you that you have:
- a blockage in your intestine (bowel obstruction).
- an opening in the wall of your stomach or intestine (bowel perforation).
- problems with food and fluid emptying from your stomach (gastric retention).
- a problem with food moving too slowly through your intestines (ileus).
- a very dilated intestine (toxic megacolon).
- an allergy to any of the ingredients in PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. See the end of this Medication Guide for a complete list of ingredients in PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
What should I tell my healthcare provider before taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?
Before taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, tell your healthcare provider about all of your medical conditions, including if you:
- have problems with serious loss of body fluid (dehydration) and changes in blood salts (electrolytes).
- have heart problems.
- have seizures or take medicines for seizures.
- have kidney problems or take medicines for kidney problems.
- have stomach or bowel problems, including ulcerative colitis.
- have problems with swallowing, gastric reflux, or if you inhale food or fluid into your lungs when eating or drinking (aspirate).
- have a condition called glucose-6-phosphate dehydrogenase (G6PD) deficiency that destroys red blood cells.
- are withdrawing from alcohol or benzodiazepines.
- are allergic to any of the ingredients in PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- are pregnant or plan to become pregnant. It is not known if PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution will harm your unborn baby.
- Talk to your healthcare provider if you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution passes into your breast milk. You and your healthcare provider should decide if you will take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution may affect how other medicines work. Do not take medicines by mouth 1 hour before or after starting each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
Especially tell your healthcare provider if you take:
- medicines to treat a blood salt (electrolyte) imbalance.
- medicines for blood pressure or heart problems.
- medicines for seizures (antiepileptics).
- medicines for kidney problems.
- water pills (diuretics).
- non-steroidal anti-inflammatory drugs (NSAID).
- laxatives. Do not take other laxatives while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- medicines for depression or other mental health problems.
- starch-based thickeners. For patients who have trouble swallowing, do not mix PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with starch-based thickeners.
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking any of the medicines listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?
See the Instructions for Use for dosing instructions. You must read, understand, and follow these instructions to take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution the right way.
- Take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution exactly as your healthcare provider tells you to take it. Your healthcare provider will tell you to take the Two-Day Split-Dosing regimen option or the One-Day Evening Only Dosing regimen option.
- On the day before the procedure, you can have breakfast, followed by a light lunch (no solid foods), and dinner of clear soup with or without plain yogurt, or plain yogurt only. You must finish dinner at least 1 hour before the start of the first PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution dose.
- Drink only clear liquids before, during, and after you take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, until 2 hours before your colonoscopy to help prevent fluid loss (dehydration).
- Do not eat solid food while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution until after your colonoscopy.
- Do not eat or drink alcohol, milk, anything colored red or purple or any other foods with pulp.
- It is important for you to drink the additional amount of clear liquids listed in the Instructions for Use.
- You may have stomach-area (abdomen) bloating after your first dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- If you have severe stomach-area (abdomen) discomfort or bloating, stop drinking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for a short time or wait a longer time between each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution until your stomach-area symptoms improve. If your stomach-area discomfort or bloating continues, tell your healthcare provider.
- If you take too much PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, call your healthcare provider
What are the possible side effects of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution? PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution can cause serious side effects, including:
-
Changes in certain blood tests . Your healthcare provider may do blood tests after you take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution to check your blood for changes. Tell your healthcare provider if you have any symptoms of too much fluid loss, including:
- vomiting
- dizziness
- feel faint, weak or lightheaded especially when you stand up (orthostatic hypotension)
- heart problems
- kidney problems
- seizures
- dry mouth
- Ulcers of the bowel or bowel problems (ischemic colitis): Tell your healthcare provider right away if you have severe stomach-area (abdomen) pain or rectal bleeding.
- Serious allergic reactions. Symptoms of a serious allergic reaction may include:
- skin rash
- swelling of the face, lips, tongue and throat
- raised red patches on your skin (hives)
- itching
- kidney problems
The most common side effects of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution include:
- anal discomfort
- thirst
- nausea
- stomach area (abdomen pain or bloating)
- sleep problems
- chills
- hunger
- discomfort
- vomiting
- indigestion
- dizziness
These are not all the possible side effects of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA- 1088.
How should I store PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?
- Store PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution that has not been mixed with water at room temperature, between 68° to 77°F (20° to 25°C).
- Store PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution that has been mixed with water in an upright position in the refrigerator.
- PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution should be taken within 24 hours after it has been mixed with water.
Keep PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution and all medicines out of the reach of children.
General information about the safe and effective use of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for a condition for which it was not prescribed. Do not give PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution to other people, even if they are going to have the same procedure you are. It may harm them.
This Medication Guide summarizes the most important information about PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider
for information that is written for healthcare professionals.
For more information, call 1-866-403-7592
What are the ingredients in PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution?
Active ingredients:
Pouch A: polyethylene glycol (PEG) 3350, sodium sulfate, sodium chloride, potassium chloride.
Pouch B: ascorbic acid and sodium ascorbate.
Inactive ingredients:
Lemon Flavor Pack: Natural lemon flavor, maltodextrin and Saccharin Sodium.
Distributed By:
Teva Pharmaceuticals USA, Inc
North Wales, PA 19454
SAP Code: 270438
Rev. 04/2022
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised: April 2022.
INSTRUCTION FOR USE
PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution
There are two different options for taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution. Your healthcare provider will tell you to take the Two-Day Split-Dosing Regimen option or the One-Day Evening Only Dosing Regimen option.
Important Information on PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution:
- You must drink all of the Dose 1 and Dose 2 of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution with either dosing regimen option. Make sure you finish Dose 2 at least 2 hours before your colonoscopy.
- After completing Dose 2, it is important that you drink additional clear liquids (including water), but you must stop drinking all liquids at least 2 hours before your colonoscopy.
- PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution must be mixed with water. Do not add any other ingredients to PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- After you start taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, you can only drink clear liquids (no solid foods) until after your colonoscopy. Examples of clear liquids include:
- water
- clear fruit juices without pulp including apple, white grape, or white cranberry
- strained limeade or lemonade
- coffee or tea (do not use any dairy or non-dairy creamer)
- clear broth
- clear soda
- gelatin (without added fruit or topping, no red or purple)
- popsicles (without pieces of fruit or pulp, no red or purple)
- Drink plenty of clear liquids before, during, and after you take PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, up until 2 hours before your colonoscopy, to help prevent fluid loss (dehydration).
- Do not eat or drink anything within 2 hours before your colonoscopy.
- Do not eat or drink alcohol, milk, anything colored red or purple or containing pulp.
- Do not take other laxatives while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- Do not take any medicines by mouth (oral) within 1 hour before or after starting each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- Do not eat any solid food while taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution until after your colonoscopy.
To take each dose of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution, you will need:
- Mixing Container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution
- One Pouch A
- One Pouch B
- One Lemon Flavor pack
- Lukewarm water
- For the Two-Day Split-Dosing Regimen:
- On the day before the colonoscopy you can eat breakfast followed by a light lunch (no solid foods). For dinner you may have a clear soup with or without plain yogurt, or plain yogurt only.
- You must finish eating at least 1 hour before you start taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- You must take the first dose between 10 to 12 hours before the second dose. The second dose must be taken at least 3 ½ hours before the colonoscopy.
- After you start taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution you can only drink clear liquids.
- For the One-Day Evening Only Dosing Regimen:
- On the day before the colonoscopy you can eat breakfast followed by a light lunch. For dinner you may have a clear soup with or without plain yogurt, or plain yogurt only.
- You must finish eating at least 1 hour before you start taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
- You must take the first dose 3 ½ hours before bedtime the evening before the colonoscopy.
- After you start taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution you can only drink clear liquids.
- Take the second dose 1 ½ hours after starting Dose 1.
Two-Day Split-Dosing Regimen Dosing Instructions
Dose 1 – Take this dose the evening before your colonoscopy (10 to 12 hours before Dose 2):
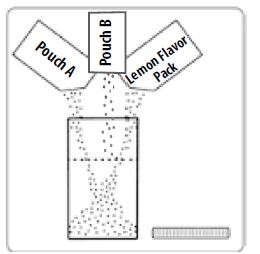
Step 1: Empty the contents of one Pouch A one Pouch B and one Lemon Flavor Pack into the Mixing Container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
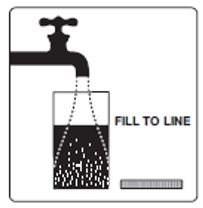
Step 2: Add lukewarm water to the Fill Line on the Mixing Container. You will need at least 32 ounces.
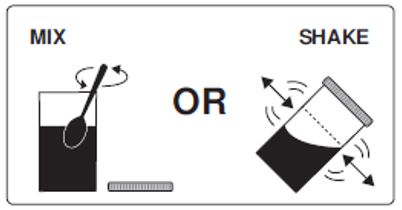
Step 3: Mix to completely dissolve the medicine from Pouch A, Pouch B and Lemon Flavor Pack into the lukewarm water. To mix the solution, stir the medicine in the Mixing Container with a spoon, or close the lid and shake.
Step 4: Drink one 8 oz. (ounce) glass of the solution every 15 minutes. Be sure to drink all of the solution in the Mixing Container. It should take about 1 hour to drink all the liquid.
If you feel like you have severe stomach pain or discomfort you can stop taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for a short period of time and then continue taking it or you can take smaller sips of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution so that you space out your dose longer than 1 hour. If you still have severe stomach pain, call your healthcare provider.
Step 5: Refill the Mixing Container with 16 oz. (at least halfway to Fill Line and enough for two 8 oz. glasses) of a clear liquid and drink all of this liquid before you go to bed.
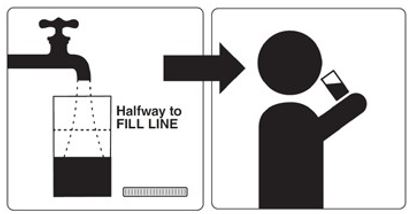
Dose 2 – Take this dose the next morning on the day of your colonoscopy (start at least 3 ½ hours before your colonoscopy):
Step 1: Repeat steps 1 through 4 from Dose 1 of the Split-Dose (2-Day) instructions.
Step 2: Fill the Mixing Container with 16 oz. (at least halfway to Fill Line and enough for two 8 oz. glasses) of a clear liquid and drink all of this liquid at least 2 hours before your colonoscopy.
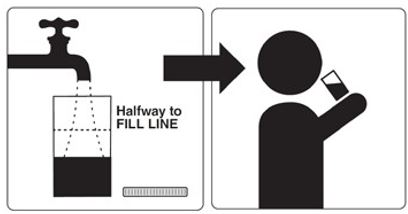
Step 3: Drink only clear liquids up to 2 hours before your colonoscopy or as prescribed by your healthcare provider. Then stop drinking liquids until after your colonoscopy.
One-Day Evening Only Dosing Regimen Instructions
Dose 1 (take at least 3 ½ hours before bedtime the evening before your colonoscopy):
Step 1: Empty the contents of one Pouch A and one Pouch B and one Lemon Flavor Pack into the Mixing Container that comes with PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution.
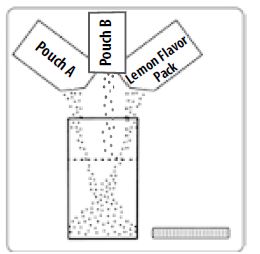
Step 2: Add lukewarm water to the Fill Line on the Mixing Container. You will need at least 32 ounces.
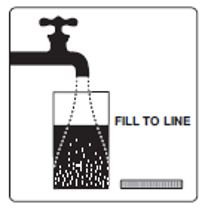
Step 3: Mix to completely dissolve the medicine from Pouch A, Pouch B and Lemon Flavor pack into the lukewarm water. To mix the solution, stir the medicine in the Mixing Container with a spoon, or close the lid and shake.
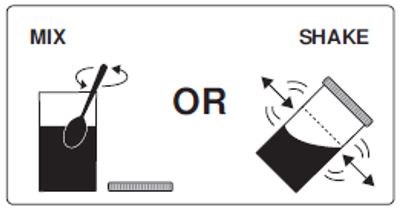
Step 4: Drink one 8 oz. (ounce) glass of the solution every 15 minutes. Be sure to drink all of the solution in the Mixing Container. It should take about 1 hour to drink all the liquid.
If you feel like you have severe stomach pain or discomfort you can stop taking PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution for a short period of time and then continue taking it or you can take smaller sips of PEG-3350, Sodium Sulfate, Sodium Chloride, Potassium Chloride, Sodium Ascorbate, and Ascorbic Acid for Oral Solution so that you space out your dose longer than 1 hour. If you still have severe stomach pain, call your healthcare provider.
Dose 2 (take about 1 ½ hours after starting Dose 1):
Step 1: Repeat Steps 1 through 4 from Dose 1 of the Evening Only (1-Day) instructions.
Step 2: After you complete steps 1 through 4, fill the Mixing Container again to the Fill Line with clear liquid and drink all of this liquid before you go to bed.
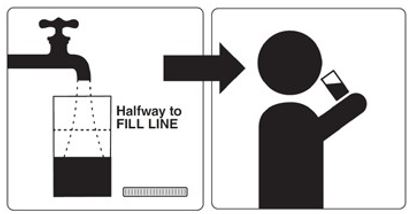
Step 3: Drink only clear liquids up to 2 hours before your colonoscopy or as prescribed by your healthcare provider. Then stop drinking liquids until after your colonoscopy.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
SAP Code: 270438
Rev. 04/2022
