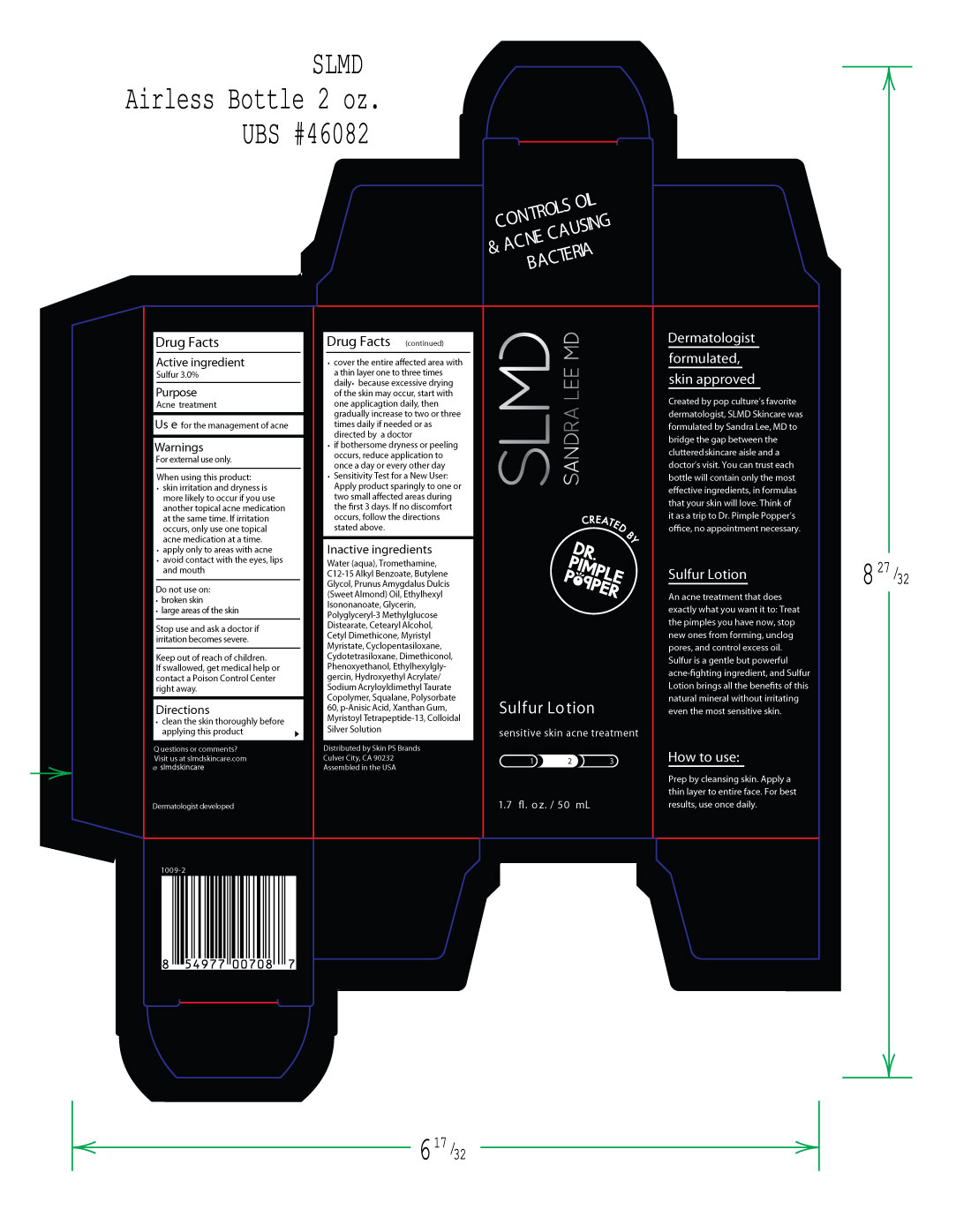
Warnings
For external use only.
When using this product:
• skin irritation and dryness is
more likely to occur if you use
another topical acne medication
at the same time. If irritation
occurs, only use one topical
acne medication at a time.
•apply only to areas with acne
• avoid contact with the eyes, lips, and mouth
Do not use on:
• broken skin
• large areas of the skin
Stop use and ask a doctor if
irriatation becomes servere.
Keep out of reach of children.
If swallowed, get medical help or
contact a Poison Control Center
right away
Directions
• clean the skin thoroughly before
applying this product
• cover the entire affected area with
a thin layer one to three times
daily • because excessive drying
of the skin may occur, start with
one applicagtion daily, then
gradually increase to two or three
times daily if needed or as
directed by a doctor
• if bothersome dryness or peeling
occurs, reduce application to
once a day or every other day
• Sensitivity Test for a New User:
Apply product sparingly to one or
two small affected areas during
the first 3 days. If no discomfort
occurs, follow the directions
stated above.
Water (aqua), Tromethamine,
C12-15 Alkyl Benzoate, Butylene
Glycol, Prunus Amygdalus Dulcis
(Sweet Almond) Oil, Ethylhexyl
Isononanoate, Glycerin,
Polyglyceryl-3 Methylglucose
Distearate, Cetearyl Alcohol,
Cetyl Dimethicone, Myristyl
Myristate, Cyclopentasiloxane,
Cydotetrasiloxane, Dimethiconol,
Phenoxyethanol, Ethylhexylgly-
gercin, Hydroxyethyl Acrylate/
Sodium Acryloyldimethyl Taurate
Copolymer, Squalane, Polysorbate
60, p-Anisic Acid, Xanthan Gum,
Myristoyl Tetrapeptide-13, Colloidal
Silver Solution