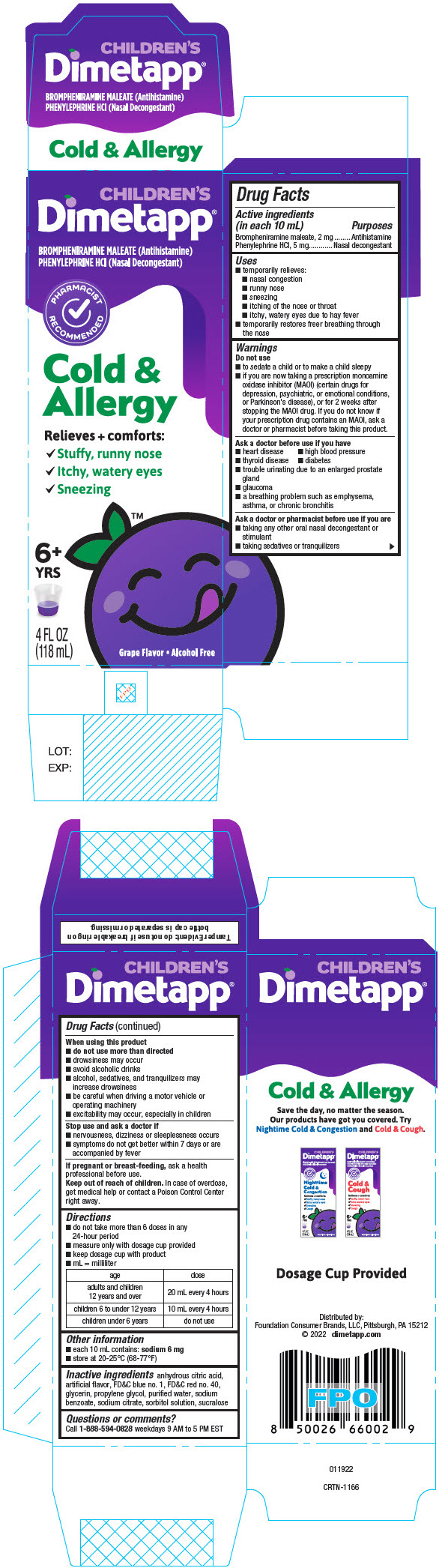
Uses
- temporarily relieves:
- nasal congestion
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes due to hay fever
- temporarily restores freer breathing through the nose
Warnings
Do not use
- to sedate a child or to make a child sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- glaucoma
- a breathing problem such as emphysema, asthma, or chronic bronchitis
Ask a doctor or pharmacist before use if you are
- taking any other oral nasal decongestant or stimulant
- taking sedatives or tranquilizers
When using this product
- do not use more than directed
- drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Directions
- do not take more than 6 doses in any 24-hour period
- measure only with dosage cup provided
- keep dosage cup with product
- mL = milliliter
| age | dose |
|---|---|
| adults and children 12 years and over | 20 mL every 4 hours |
| children 6 to under 12 years | 10 mL every 4 hours |
| children under 6 years | do not use |
Inactive ingredients
anhydrous citric acid, artificial flavor, FD&C blue no. 1, FD&C red no. 40, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol solution, sucralose
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
CHILDREN'S
Dimetapp®
BROMPHENIRAMINE MALEATE (Antihistamine)
PHENYLEPHRINE HCl (Nasal Decongestant)
PHARMACIST
RECOMMENDED
Cold &
Allergy
Relieves + comforts:
- ✔
- Stuffy, runny nose
- ✔
- Itchy, watery eyes
- ✔
- Sneezing
6+
YRS
4 FL OZ
(118 mL)
Grape Flavor • Alcohol Free