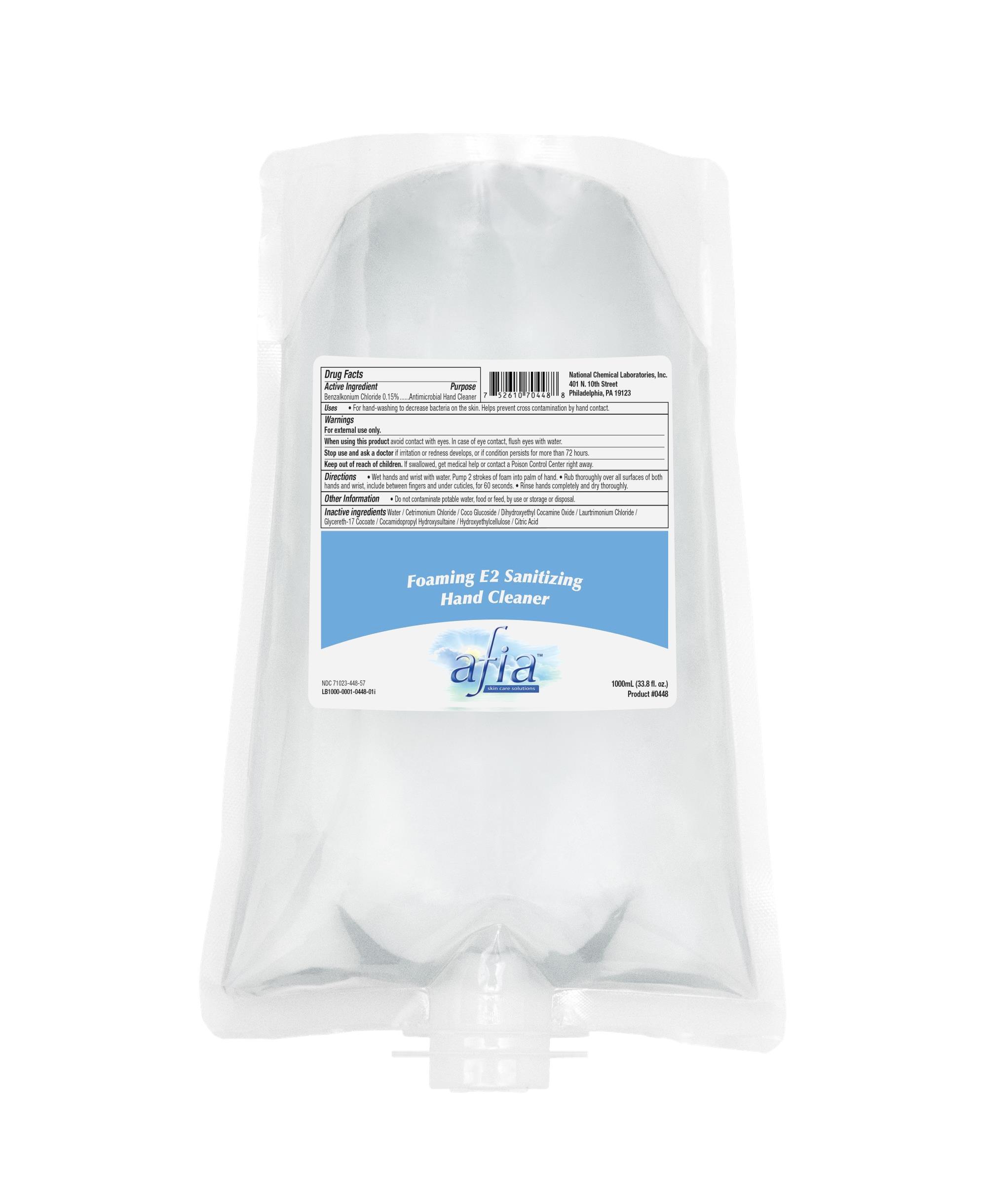
Drug Facts
Active Ingredient. Purpose
Benzalkonium Chloride 0.15%...........Antimicrobial hand Cleaner

Uses
- Uses • For hand-washing to decrease bacteria on the skin. Helps prevent cross contamination by hand contact.

Warnings:
Warnings For external use only.
When using this product avoid contact with eyes. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation or redness develops, or if condition persists for more than 72 hours.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.

Directions
• Wet hands and wrist with water. Pump 2 strokes of foam into palm of hand. • Rub thoroughly over all surfaces of both hands and wrist, include between fingers and under cuticles, for 60 seconds. • Rinse hands completely and dry thoroughly.

Inactive ingredients
Water / Cetrimonium Chloride / Coco Glucoside / Dihydroxyethyl Cocamine Oxide / Laurtrimonium Chloride / Glycereth-17 Cocoate / Cocamidopropyl Hydroxysultaine / Hydroxyethylcellulose / Citric Acid

Uses
For hand-washing to decrease bacteria on the skin. Helps prevent cross contamination by hand contact.