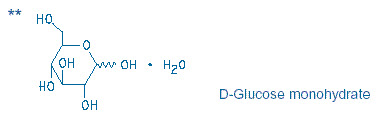FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Potassium Chloride in Dextrose Injection is indicated as a source of water, electrolytes and calories.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- •
- Potassium Chloride in Dextrose Injection is only for intravenous infusion [see Warnings and Precautions (5.2)].
- •
- For patients receiving Potassium Chloride in Dextrose Injection at greater than maintenance rates, frequent monitoring of serum potassium concentrations and serial electrocardiograms (ECGs) are recommended.
- •
- The osmolarity of Potassium Chloride in Dextrose Injection is 293 mOsmol/L (calc). Peripheral administration is generally acceptable; however; consider central vein administration if there is peripheral vein irritation, phlebitis, and/or associated pain.
- •
- Do not administer Potassium Chloride in Dextrose Injection simultaneously with blood products through the same administration set because of the possibility of pseudo agglutination or hemolysis.
- •
- To prevent air embolism, use a non-vented infusion set or close the vent on a vented set, avoid multiple connections, do not connect flexible containers in series, fully evacuate residual gas in the container prior to administration, do not pressurize the flexible container to increase flow rates, and if administration is controlled by a pumping device, turn off pump before the container runs dry.
- •
- Prior to infusion, visually inspect the solution for particulate matter. The solution should be clear and there should be no precipitates. Do not administer unless solution is clear and container is undamaged.
- •
- Use of a final filter is recommended during administration of parenteral solutions, where possible.
2.2 Recommended Dosage
The infusion rate and volume depends on the age, weight, clinical and metabolic conditions of the patient and concomitant therapy. Electrolyte supplementation may be indicated according to the clinical needs of the patient.
The administration rate should be governed, especially for premature infants with low birth weight, during the first few days of therapy, by the patient’s tolerance to dextrose. Increase the infusion rate gradually as indicated by frequent monitoring of blood glucose concentrations [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
2.3 Instructions for Use
To Open
- •
- Do not remove container from overwrap until ready to use.
- •
- Tear overwrap down side at slit and remove solution container.
- •
- Visually inspect the container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Evaluate the following:
- o
- If the outlet port protector is damaged, detached, or not present, discard container.
- o
- Check to ensure the solution is clear and there are no precipitates. Discard if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals.
- o
- Check for minute leaks by squeezing inner bag firmly. If leaks are found, discard container.
Preparation for Administration
To Add Medication
- •
- Additives may be incompatible. Complete information is not available. Do not use additives known or determined to be incompatible.
- •
- Before adding a substance or medication, verify that it is soluble and/or stable in Potassium Chloride in Dextrose Injection and that the pH range of Potassium Chloride in Dextrose Injection is appropriate.
- •
- Consult with pharmacist, if available. If, in the informed judgment of the healthcare provider, it is deemed advisable to introduce additives, use aseptic technique.
- •
- When introducing additives, consult the instructions for use of the medication to be added and other relevant literature.
To Add Medication Before Solution Administration
- 1.
- Prepare medication site.
- 2.
- Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
- 3.
- Mix solution and medication thoroughly. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
- 4.
- After addition, check to ensure the solution is clear and there are no precipitates. Discard if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals.
To Add Medication During Solution Administration
- 1.
- Close clamp on the set.
- 2.
- Prepare medication site.
- 3.
- Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
- 4.
- Remove container from IV pole and/or turn to an upright position.
- 5.
- Evacuate both ports by squeezing them while container is in the upright position.
- 6.
- Mix solution and medication thoroughly.
- 7.
- After addition, check to ensure the solution is clear and there are no precipitates. Discard if there is a color change and/or the appearance of precipitates, insoluble complexes or crystals, do not use.
- 8.
- Return container to in use position and continue administration.
Storage
- •
- Use promptly; do not store solutions containing additives.
- •
- Single-dose container.
- •
- Discard any unused portion.
3 DOSAGE FORMS AND STRENGTHS
Potassium Chloride in Dextrose Injection, USP is a clear solution of 20 mEq Potassium Chloride and 5% Dextrose in a 1000 mL single-dose, flexible container.
4 CONTRAINDICATIONS
Potassium Chloride in Dextrose Injection is contraindicated in patients with:
- •
- known hypersensitivity to potassium chloride and/or dextrose [see Warnings and Precautions 5.1)]
- •
- clinically significant hyperkalemia [see Warnings and Precautions (5.2)]
- •
- clinically significant hyperglycemia [see Warnings and Precautions (5.3)]
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity and infusion reactions, including anaphylaxis, have been reported with Potassium Chloride in Dextrose Injection [see Adverse Reactions (6)]. Stop the infusion immediately if signs or symptoms of a hypersensitivity or infusion reaction develops [see Contraindications (4)]. Appropriate therapeutic countermeasures must be instituted as clinically indicated.
5.2 Hyperkalemia
Potassium-containing solutions, including Potassium Chloride in Dextrose Injection may increase the risk of hyperkalemia. Hyperkalemia can be asymptomatic and manifest only by increased serum potassium concentrations and/or characteristic electrocardiographic (ECG) changes. Cardiac arrhythmias, some fatal, can develop at any time during hyperkalemia.
To avoid life threatening hyperkalemia, do not administer Potassium Chloride in Dextrose Injection as an intravenous push (i.e., intravenous injection manually with a syringe connected to the intravenous access, without quantitative infusion device [see Dosage and Administration (2.1)].
Patients at increased risk of developing hyperkalemia and cardiac arrhythmias include those:
- •
- with severe renal impairment, acute dehydration, extensive tissue injury or burns, and certain cardiac disorders such as congestive heart failure or AV block (especially if they receive digoxin).
- •
- with hyperosmolality, acidosis, or undergoing correction of alkalosis (conditions associated with a shift of potassium from intracellular to extracellular space).
- •
- treated concurrently or recently with agents or products that can cause or increase the risk of hyperkalemia [see Drug Interactions (7.1)].
Avoid use of Potassium Chloride in Dextrose Injection in patients with, or at risk for, hyperkalemia. If use cannot be avoided, use a product with a low amount of potassium chloride, infuse slowly and monitor serum potassium concentrations and ECGs.
5.3 Hyperglycemia and Hyperosmolar Hyperglycemic State
The use of dextrose infusions in patients with impaired glucose tolerance may worsen hyperglycemia. Administration of dextrose at a rate exceeding the patient’s utilization rate may lead to hyperglycemia, coma, and death.
Hyperglycemia is associated with an increase in serum osmolality, resulting in osmotic diuresis, dehydration and electrolyte losses [see Warnings and Precautions (5.6)]. Patients with underlying central nervous system disease and renal impairment who receive dextrose infusions, may be at greater risk of developing hyperosmolar hyperglycemic state.
Monitor blood glucose concentrations and treat hyperglycemia to maintain concentrations within normal limits while administering Potassium Chloride in Dextrose Injection. Insulin may be administered or adjusted to maintain optimal blood glucose concentrations.
5.4 Hyponatremia
Potassium Chloride in Dextrose Injection is an isotonic solution [see Description, Table 1 (11)]. In the body, however, glucose containing fluids can become extremely physiologically hypotonic due to rapid glucose metabolization. Monitoring of serum sodium is particularly important for hypotonic fluids.
Depending on the tonicity of the solution, the volume and rate of infusion, and depending on a patient’s underlying clinical condition and capability to metabolize glucose, intravenous administration of glucose can cause electrolyte disturbances, most importantly hypo- or hyperosmotic hyponatremia.
The risk for hyponatremia is increased, in pediatric patients, elderly patients, postoperative patients, those with psychogenic polydipsia and in patients treated with medications that increase the risk of hyponatremia (such as certain diuretic, antiepileptic and psychotropic medications). Close clinical monitoring may be warranted.
Acute hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy and vomiting. Patients with brain edema are at particular risk of severe, irreversible and life-threatening brain injury. Patients at increased risk for developing complications of hyponatremia, such as hyponatremic encephalopathy include pediatric patients; women, in particular, premenopausal women; patients with hypoxemia; and in patients with underlying central nervous system disease [see Use in Specific Populations (8.4, 8.5)].
Rapid correction of hyponatremia is potentially dangerous with risk of serious neurologic complications such as osmotic demyelination syndrome with risk of seizures and cerebral edema. To avoid complications, monitor serum sodium and chloride concentrations, fluid status, acid-base balance, and signs of neurologic complications.
High volume infusion must be used with close monitoring in patients with cardiac or pulmonary failure, and in patients with non-osmotic vasopressin release (including SIADH), due to the risk of hospital-acquired hyponatremia.
5.5 Hypokalemia
Infusion of Potassium Chloride in Dextrose Injection may result in hypokalemia, leading to arrhythmias, muscle weakness, paralysis, heart block, and rhabdomyolysis.
Hypokalemic periodic paralysis, metabolic alkalosis, increased gastrointestinal losses (e.g., diarrhea, vomiting), prolonged low potassium diet or primary hyperaldosteronism may increase the risk of hypokalemia. If use cannot be avoided, monitor serum potassium levels.
5.6 Fluid Overload
Depending on the volume and rate of infusion, the patient’s underlying clinical condition and capability to metabolize dextrose, intravenous administration of Potassium Chloride in Dextrose Injection can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
Avoid Potassium Chloride in Dextrose Injection in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, monitor fluid balance, electrolyte concentrations, and acid-base balance as needed and especially during prolonged use.
5.7 Refeeding Syndrome
Refeeding severely undernourished patients may result in the refeeding syndrome that is characterized by the shift of potassium, phosphorus, and magnesium intracellularly as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. To prevent these complications, monitor severely undernourished patients and slowly increasing nutrient intake.
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of Potassium Chloride in Dextrose Injection were identified in post marketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Hypersensitivity reactions: including anaphylaxis and chills [see Warnings and Precautions (5.1)].
- •
- Hyperkalemia, including cardiac arrest, as a manifestation [see Warnings and Precautions (5.2)]
- •
- Hyponatremia and hyponatremic encephalopathy [see Warnings and Precautions (5.4)]
- •
- Hypokalemia [see Warnings and Precautions (5.5)]
- •
- Hypervolemia [see Warnings and Precautions (5.6)]
- •
- Injection site reactions: infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation, infusion site rash, infusion site pain, infusion site vesicles, infusion site pruritus, pyrexia and chills
7 DRUG INTERACTIONS
7.1 Other Products that Cause Hyperkalemia
Administration of Potassium Chloride in Dextrose Injection in patients treated concurrently or recently with other products that can cause hyperkalemia or increase the risk of hyperkalemia (e.g., potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers) increases the risk of severe and potentially fatal hyperkalemia, in particular in the presence of other risk factors for hyperkalemia [see Warnings and Precautions (5.2)]. Avoid use of Potassium Chloride in Dextrose Injection in patients receiving such products. If use cannot be avoided, monitor serum potassium concentrations.
7.2 Other Products that Affect Glycemic Control, Vasopressin or Fluid and/or Electrolyte Balance
Potassium Chloride in Dextrose Injection can affect glycemic control, vasopressin and fluid and/or electrolyte balance [see Warnings and Precautions (5.3, 5.4, 5.5, 5.6)]. Monitor blood glucose concentrations, fluid balance, serum electrolyte concentrations and acid-base balance when using Potassium Chloride in Dextrose Injection in patients treated with other substances that affect glycemic control, vasopressin or fluid and/or electrolyte balance.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Appropriate administration of Potassium Chloride in Dextrose Injection during pregnancy is not expected to cause adverse developmental outcomes, including congenital malformations. Animal reproduction studies have not been conducted with Potassium Chloride in Dextrose Injection.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
Potassium is present in human breast milk. There are no data on the effects of Potassium Chloride in Dextrose Injection on a breastfed infant or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Potassium Chloride in Dextrose Injection and any potential adverse effects on the breastfed infant from Potassium Chloride in Dextrose Injection or from the underlying maternal condition.
8.4 Pediatric Use
The safety profile of Potassium Chloride in Dextrose Injection in pediatric patients is similar to adults.
Neonates, especially premature infants with low birth weight, are at increased risk of developing hypo- or hyperglycemia and therefore need close monitoring during treatment with intravenous glucose solutions to ensure adequate glycemic control in order to avoid potential long term adverse effects.
Closely monitor plasma electrolyte concentrations in pediatric patients who may have impaired ability to regulate fluids and electrolytes. In very low birth weight infants, excessive or rapid administration of Potassium Chloride in Dextrose Injection may result in increased serum osmolality and risk of intracerebral hemorrhage.
Children (including neonates and older children) are at increased risk of developing hyponatremia as well as for developing hyponatremic encephalopathy.
8.5 Geriatric Use
Potassium Chloride in Dextrose Injection is known to be substantially excreted by the kidney, and the risk of adverse reactions to this product may be greater in patients with impaired renal function [see Warnings and Precautions (5.2, 5.3)].
Elderly patients are at increased risk of developing hyponatremia as well as for developing hyponatremic encephalopathy [see Warnings and Precautions (5.4)].
Dose selection for an elderly patient should be cautious, starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Administration of Potassium Chloride in Dextrose Injection in patients with renal impairment may result in hyperkalemia, hyponatremia, and/or fluid overload. Monitor patients with renal impairment for development of these adverse reactions [see Warnings and Precautions (5.2, 5.4, 5.6)].
10 OVERDOSAGE
Excess administration of Potassium Chloride in Dextrose Injection can cause:
- Hyperkalemia
- •
- Manifestations of hyperkalemia may include:
- o
- disturbances in cardiac conduction and arrhythmias, including bradycardia, heart block, asystole, ventricular tachycardia, ventricular fibrillation, and ECG changes (peaking of T waves, loss of P waves, and QRS widening)
- o
- hypotension,
- o
- muscle weakness up to and including muscular and respiratory paralysis, paresthesia of extremities,
- o
- gastrointestinal symptoms (ileus, nausea, vomiting, abdominal pain)
- The presence of any ECG findings that are suspected to be caused by hyperkalemia should be considered a medical emergency.
- If hyperkalemia is present or suspected, discontinue the infusion immediately and institute close ECG, laboratory and other monitoring and, as necessary, corrective therapy to reduce serum potassium concentrations [see Warnings and Precautions (5.2)].
- Other Electrolyte and Fluid Disorders
- •
- Hyperglycemia, hyperosmolality, and adverse effects on water and electrolyte balance, and corresponding complications, which can be fatal [see Warnings and Precautions (5.3, 5.6)].
- •
- Hyponatremia, manifestations may include seizures, coma, cerebral edema and death) [see Warnings and Precautions (5.4)].
- •
- Fluid overload (which can lead to central and/or peripheral edema) [see Warnings and Precautions (5.6)].
- •
- Hypernatremia, especially in patients with severe renal impairment.
Interventions include discontinuation of the infusion, dose reduction, monitoring of fluid balance, electrolyte concentrations and acid-base balance and institution of appropriate corrective measures such as administration of exogenous insulin.
11 DESCRIPTION
Potassium Chloride in Dextrose Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment and caloric supply in a single-dose container for intravenous administration. It contains no antimicrobial agents. Composition, osmolarity, pH, ionic concentration and caloric content are shown in Table 1.
|
||||||||
|
Table 1 |
Size (mL) |
Composition (g/L) |
*Osmolarity (mOsmol/L) |
pH |
Ionic Concentration (mEq/L) |
Caloric Content (kcal/L) |
||
|
**Dextrose Hydrous, USP |
Potassium Chloride, USP |
Potassium |
Chloride |
|||||
|
Potassium Chloride in |
||||||||
|
mEq Potassium |
||||||||
|
20 mEq |
1000 |
50 |
1.5 |
293 |
4.5 |
20 |
20 |
170 |
Dextrose is derived from corn.
The VIAFLEX Plus plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). VIAFLEX Plus on the container indicates the presence of a drug additive in a drug vehicle. The VIAFLEX Plus plastic container system utilizes the same container as the VIAFLEX plastic container system. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
16 HOW SUPPLIED/STORAGE AND HANDLING
Potassium Chloride in 5% Dextrose Injection, is a clear solution in 1000 mL single-dose, flexible containers available as follows:
|
Code |
Size (mL) |
NDC |
Product Name |
|
2B1134 |
1000 |
0338-0683-04 |
20 mEq Potassium Chloride |
Storage: Avoid excessive heat. Store at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
17 PATIENT COUNSELING INFORMATION
Inform patients, caregivers or home healthcare providers of the following risks of Potassium Chloride in Dextrose Injection:
- •
- Hypersensitivity reactions [see Warnings and Precautions (5.1)]
- •
- Hyperkalemia [see Warnings and Precautions (5.2)]
- •
- Hyperglycemia and hyperosmolar hyperglycemic state [see Warnings and Precautions (5.3)]
- •
- Hyponatremia [see Warnings and Precautions (5.4)]
- •
- Hypokalemia [see Warnings and Precautions (5.5)]
- •
- Fluid overload [see Warnings and Precautions (5.6)]
- •
- Refeeding syndrome [see Warnings and Precautions (5.7)]
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
Distributed in Canada by
Baxter Corporation
Mississauga, ON L5N 0C2
07-19-75-361
Baxter and Viaflex are trademarks of Baxter International Inc.
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
2B1134
NDC 0338-0683-04
DIN 00438049
20 mEq
Potassium Chloride
(20 mEq/L)
Potassium Chloride in
5% Dextrose Injection USP
1000 mL
EACH 100 mL CONTAINS 5 g DEXTROSE HYDROUS USP
150 mg POTASSIUM CHLORIDE USP pH 4.5 (3.5 TO 6.5)
mEq/L POTASSIUM 20 CHLORIDE 20 OSMOLARITY 293
mOsmol/L (CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER ADDITIVES MAY BE INCOMPATIBLE CONSULT WITH
PHARMACIST IF AVAILABLE WHEN INTRODUCING ADDITIVES USE
ASEPTIC TECHNIQUE MIX THOROUGHLY DO NOT STORE
DOSAGE INTRAVENOUSLY AS DIRECTED BY A PHYSICIAN SEE
DIRECTIONS CAUTIONS SQUEEZE AND INSPECT INNER BAG
WHICH MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE
FOUND MUST NOT BE USED IN SERIES CONNECTIONS DO NOT
ADMINISTER SIMULTANEOUSLY WITH BLOOD DO NOT USE
UNLESS SOLUTION IS CLEAR RX ONLY STORE UNIT IN
MOISTURE BARRIER OVERWRAP AT ROOM TEMPERATURE
(25°C/77°F) UNTIL READY TO USE AVOID EXCESSIVE HEAT
SEE INSERT
VIAFLEX PLUS CONTAINER PL 146 PLASTIC
BAXTER VIAFLEX AND PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INC
FOR PRODUCT INFORMATION 1-800-933-0303
Baxter Logo
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA
DISTRIBUTED IN CANADA BY
BAXTER CORPORATION
TORONTO ONTARIO CANADA