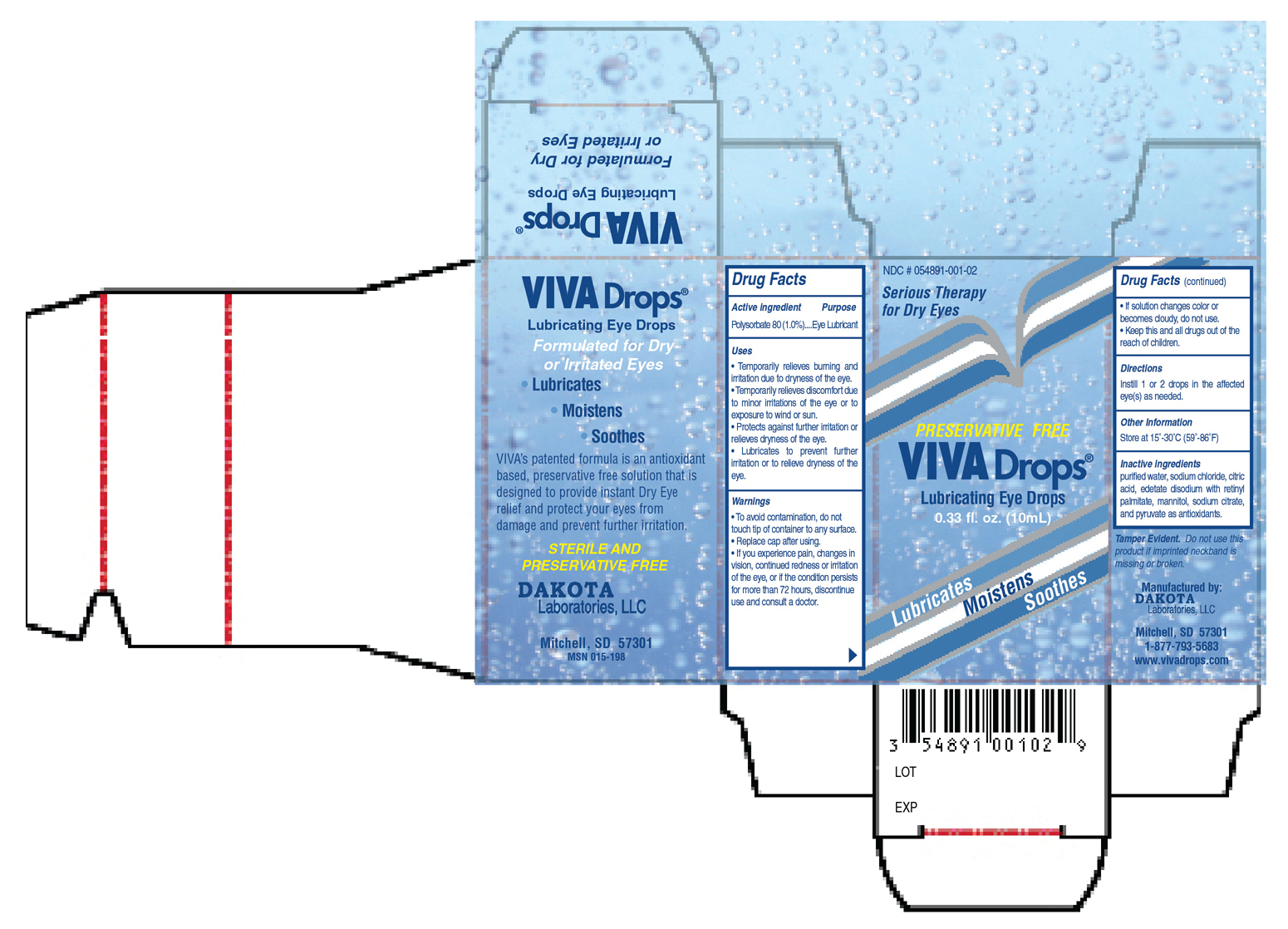
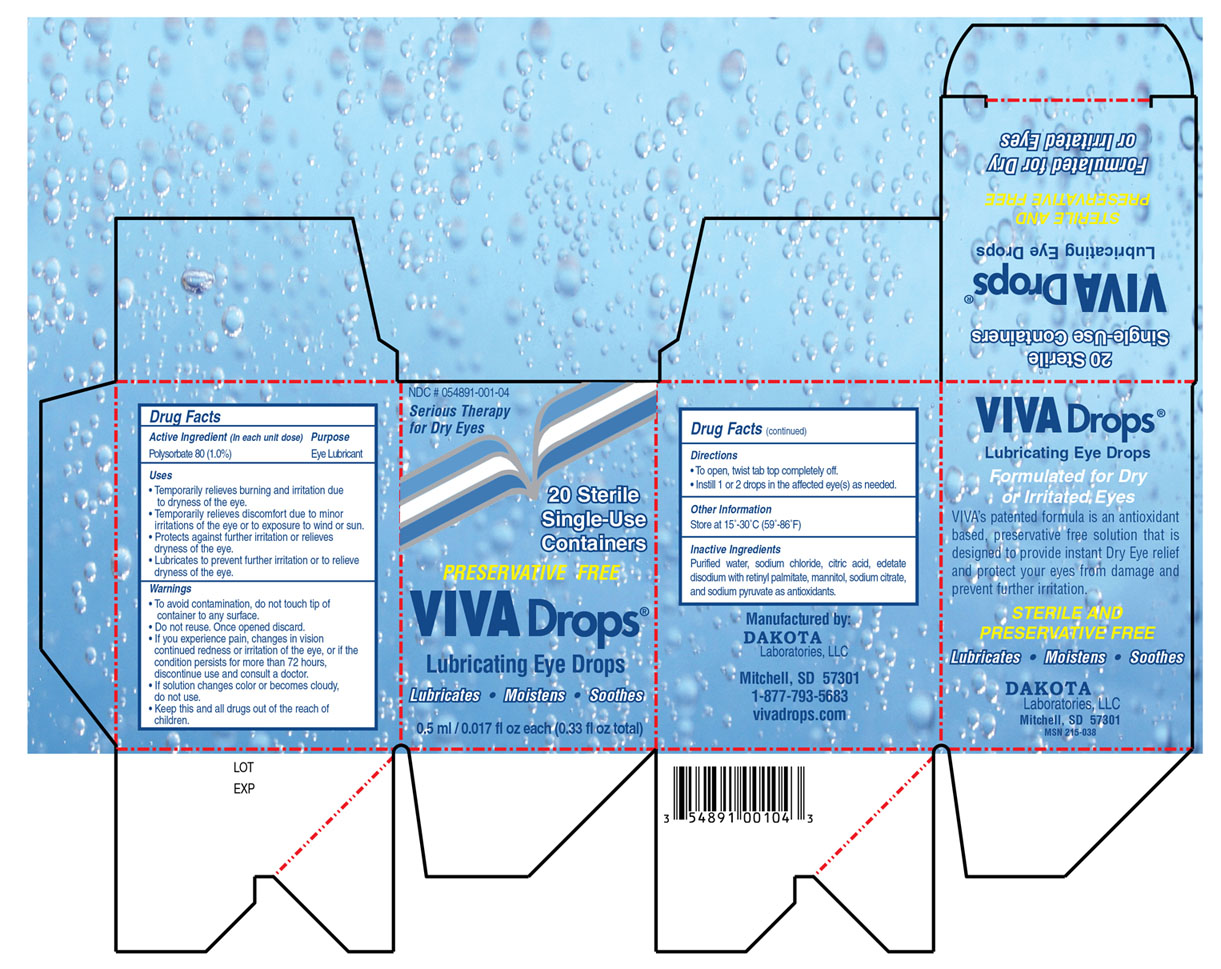
• Temporarily relieves burning and irritation due to dryness of the eye.
• Temporarily relieves discomfort due to minor irritations of the eye or to exposure to wind or sun.
• Protects against further irritation or relieves dryness of the eye.
• Lubricates to prevent further irritation or to relieve dryness of the eye.
• To avoid contamination, do not touch tip of container to any surface.
• Replace cap after using
• If you experience pain, changes in vision, continued redness or irritation of the eye, or if the condition persists for more than 72 hours, discontinue use and consult a doctor.
• If solution changes color or becomes cloudy, do not use.