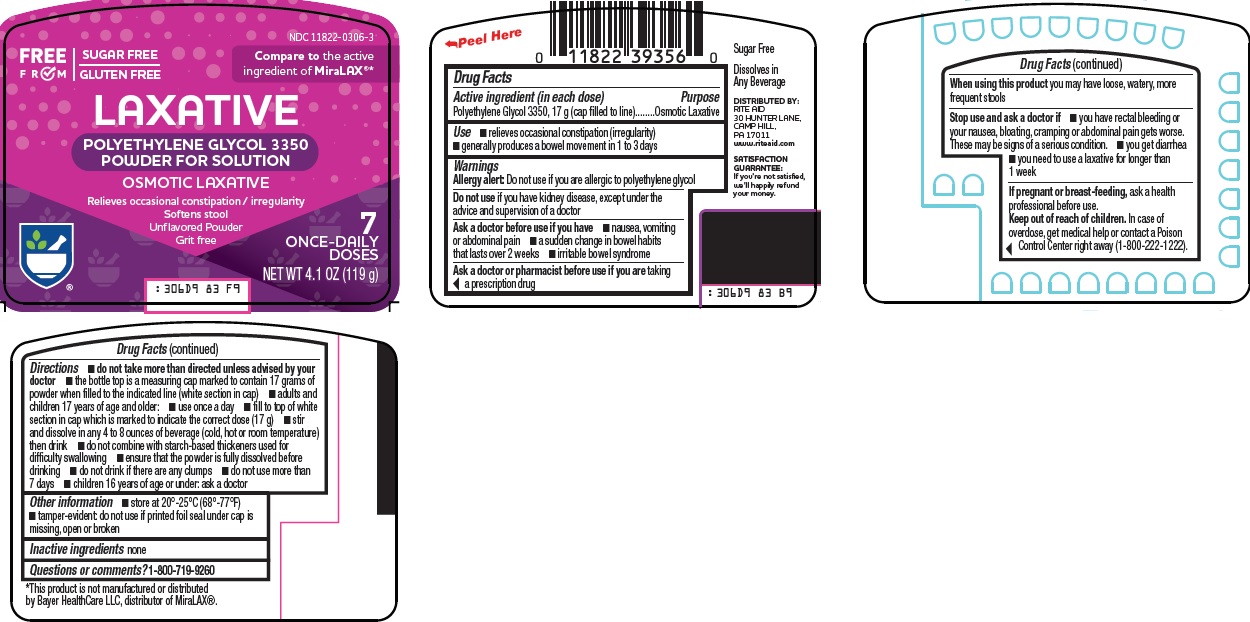
Use
- •
- relieves occasional constipation (irregularity)
- •
- generally produces a bowel movement in 1 to 3 days
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol
Ask a doctor before use if you have
- •
- nausea, vomiting or abdominal pain
- •
- a sudden change in bowel habits that lasts over 2 weeks
- •
- irritable bowel syndrome
Directions
- •
- do not take more than directed unless advised by your doctor
- •
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- •
- adults and children 17 years of age and older:
- •
- use once a day
- •
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- •
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- •
- do not combine with starch-based thickeners used for difficulty swallowing
- •
- ensure that the powder is fully dissolved before drinking
- •
- do not drink if there are any clumps
- •
- use no more than 7 days
- •
- children 16 years of age or under: ask a doctor
Other information
- •
- store at 20˚-25˚C (68˚-77˚F)
- •
- tamper-evident: do not use if printed foil seal under cap is missing, open or broken
Principal Display Panel
FREE FROM
SUGAR FREE
GLUTEN FREE
Compare to the active ingredient of MiraLAX®
LAXATIVE
POLYETHYLENE GLYCOL 3350 POWDER FOR SOLUTION
OSMOTIC LAXATIVE
Relieves occasional constipation / irregularity
Softens stool
Unflavored Powder
Grit Free
7 ONCE-DAILY DOSES
NET WT 4.1 OZ (119 g)