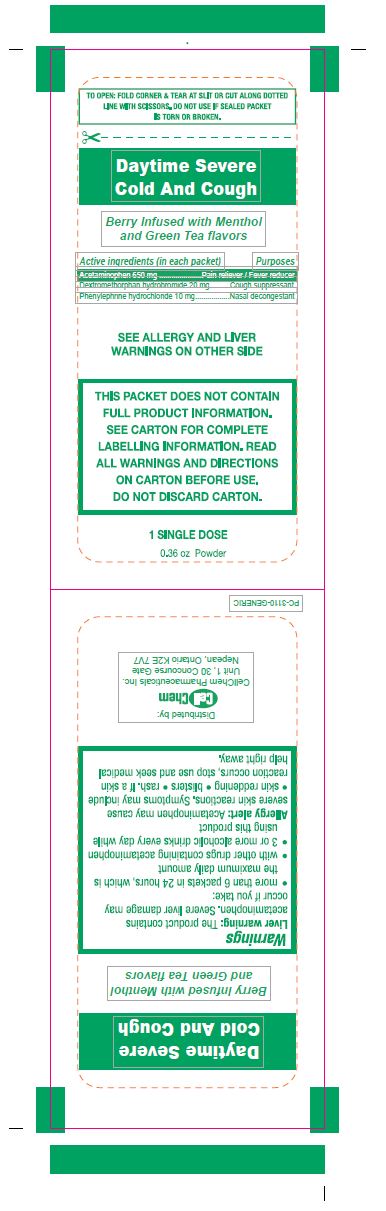
DAYTIME SEVERE COLD AND COUGH- acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride powder
Cellchem Pharmaceuticals Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Daytime Severe
Cold And Cough
Drug Facts
Active Ingredients (in each packet) Purpose
Acetaminophen 650 mg.........................................Pain reliever/Fever reducer
Dextromethorphan hydrobromide 20 mg................................Cough Suppressant
Phenylephrine Hydrochloride 10 mg......................................Nasal decongestant
Uses
- •
- Temporarily relieves these symptoms due to cold:
- o
- minor aches and pains
- o
- minor sore throat pain
- o
- headache
- o
- nasal and sinus congestion
- o
- cough due to minor throat and bronchial irritation
- •
- Temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- •
- more than 6 packets in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product.
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include
- •
- skin reddening
- •
- blisters
- •
- rash. If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- in children under 12 years of age
- •
- if you are allergic to acetaminophen
- •
- with any other drug containing acetaminophen (prescription and non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains a MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- liver disease
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Ask a doctor or pharmacist before use if you are
- •
- taking the blood thinning drug warfarin
Stop use and ask a doctor if
- •
- nervousness, dizziness, or sleeplessness occurs
- •
- fever gets worse or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- pain, cough or nasal congestion gets worse or lasts more than 7 days
- •
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Directions
- •
- do not use more than directed
- •
- take every 4 hours, while symptoms persist.
Do not take more than 6 packets in 24 hours unless directed by a doctor.
|
Age |
Dose |
|
adults and children 12 years and over |
one packet |
|
children under 12 years |
do not use |
- •
- dissolve contents of one packet into 8 oz. hot water; sip while hot. Consume entire drink within 10-15 minutes.
- •
- if using a microwave, add contents of one packet to 8 oz. of cool water; stir briskly before and after heating. Do not overheat.
Other Information
- •
- each packet contains: potassium 10 mg, sodium 24 mg.
- •
- phenylketonurics: contains phenylalanine 14 mg per packet.
- •
- store at controlled room temperature 20-25°C (68 -77°F). Protect from heat and moisture.
Inactive ingredients
Acesulfame potassium, anhydrous citric acid, aspartame, FD&C Blue No.1, FD&C red No.40, maltodextrin, menthol, natural flavors, sodium citrate, starch and sugar.
| DAYTIME SEVERE COLD AND COUGH
acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride powder |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Cellchem Pharmaceuticals Inc (111518618) |
| Registrant - Cellchem Pharmaceuticals Inc (111518618) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Laboratoires Confab Inc | 241754217 | MANUFACTURE(73147-3110) | |