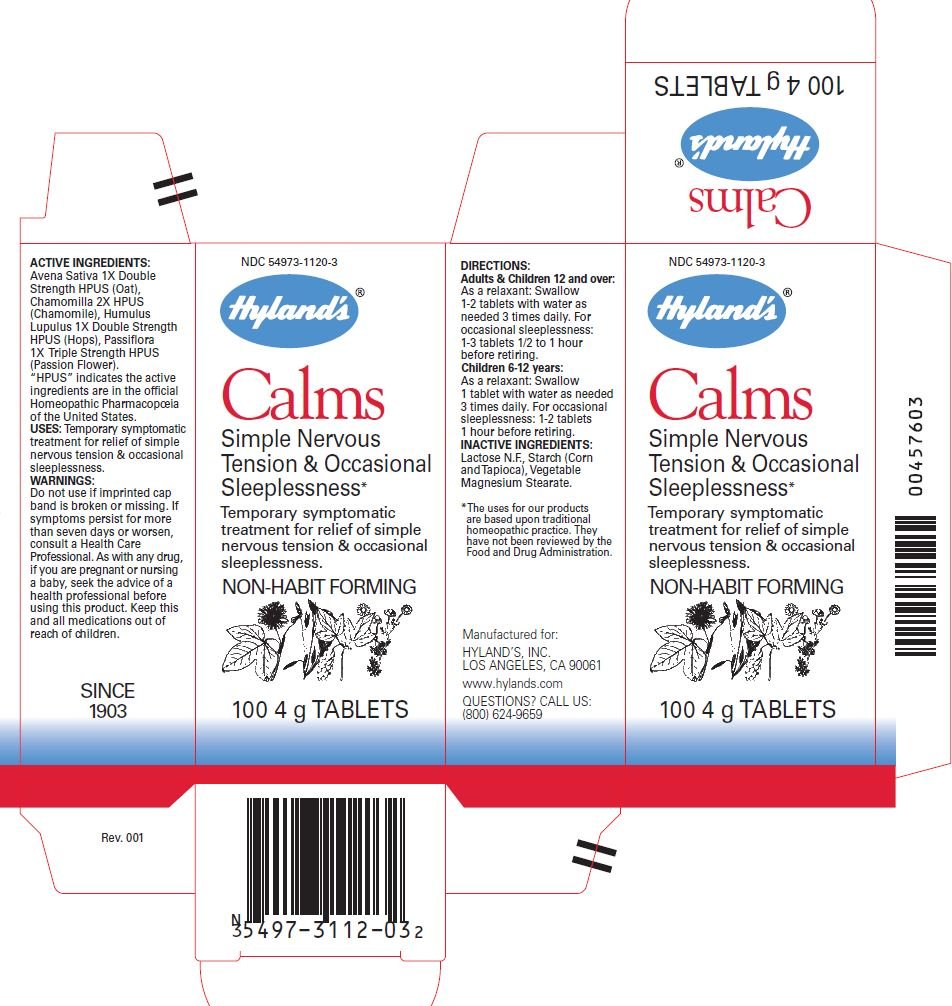
CALMS NERVE TENSION SLEEPLESSNESS- passiflora incarnata flower, avena sativa leaf, hops, and chamomile tablet
Hyland's Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Temporary symptomatic treatment for relief of simple nervous tension & occasional sleeplessness.
HOMEOPATHIC FORMULA
PASSIFLORA 1X Triple Strength HPUS (Passion Flower)
AVENA SATIVA 1X Double Strength HPUS (Oat)
HUMULUS LUPULUS 1X Double Strength HPUS (Hops)
CHAMOMILLA 2X HPUS (Chamomile)
“HPUS” indicates the active
ingredients are in the official
Homeopathic Pharmacopoeia
of the United States.
Lactose N.F.,
Starch (Corn and Tapioca),
Vegetable Magnesium Stearate.
WARNING
Do not use if imprinted cap band is missing or broken.
If symptoms persist for more than seven days or worsen, consult a Health Care Professional.
As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
Keep this and all medications out of reach of children.
DIRECTIONS
Adults & Children 12 and over:
As a relaxant: Swallow 1-2 tablets with water as needed 3 times daily. For occasional sleeplessness:
1-3 tablets 1/2 to 1 hour before retiring.
Children 6-12 years:
As a relaxant: 1 tablet with water as needed 3 times daily. For occasional sleeplessness: 1-2 tablets 1 hour before retiring.
QUESTIONS?
CALL US:
(800) 624-9659
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Carton
NDC 54973-1120-3
Hyland's®
Calms
Simple Nerve Tension
& Occasional Sleeplessness*
Temporary symptomatic
treatment for relief of simple
nervous tension & occasional
sleeplessness.
NON-HABIT FORMING
100 4 g TABLETS
Hyland's Inc.
