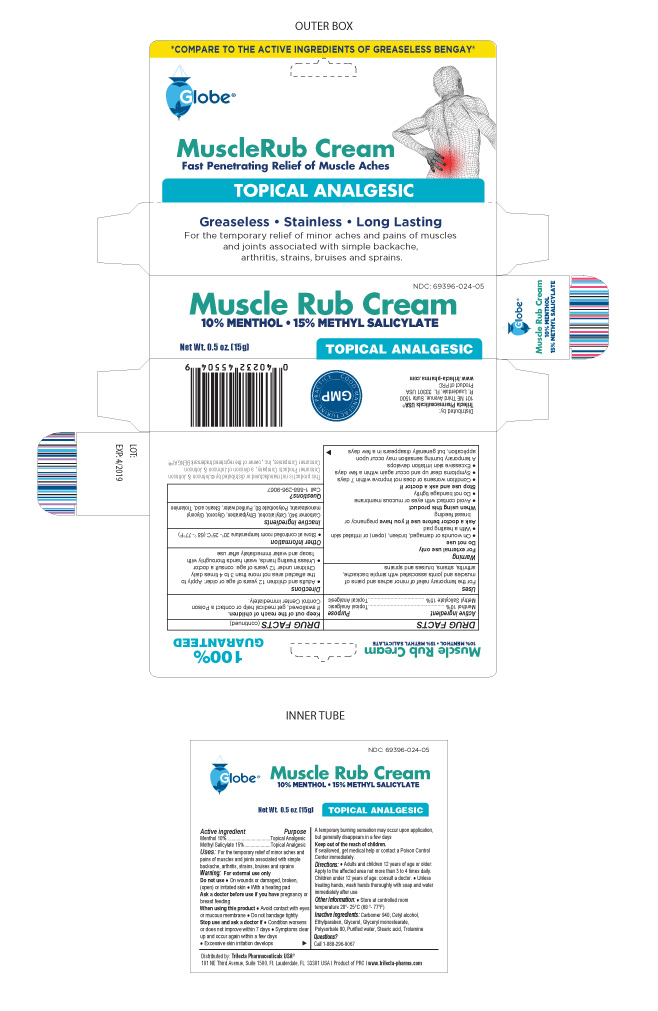
For the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains
Warnings
For external use only
Do not use:
- On wounds or damaged, broken, (open) or irritated skin
- With a heating pad
Ask a doctor before use if you are pregnant or breast feeding.
When using this product
- Avoid contact with eyes or mucous membrane
- Do not bandage tightly
Stop Use and ask a doctor
- Conditions worsens or does not improve within 7 days
- Symptoms clear up and occur again within a few days
- Excessive skin irritation develops
A temporary burning sensation may occur upon application, but generally disappears in a few days
Keep out of the reach of Children
If swallowed, get medical help or contact a Poison Control Center immediately
Directions
- Adults and children 12 years of age or older. Apply to the affected area not more than 3 to 4 times daily.
Children under 12 years of age. consult a doctor
- Unless treating hands, wash hands thoroughly with soap and water immediately after use.
Other Information
- Store at controlled room temperature 20º-25ºC (68º-77ºF)
Questions ? Call 1-888-296-9067
Inactive Ingredients
Carbomer 940, Cetyl alcohol, Glycerol, Glyceryl monostearate, Methylparaben, Polysorbate 80, Propylparaben, Purified Water, Stearic acid, Trolamine