DESCRIPTION
Methocarbamol Injection, USP a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant with sedative and musculoskeletal relaxant properties. It is a sterile, pyrogen-free solution intended for intramuscular or intravenous administration.
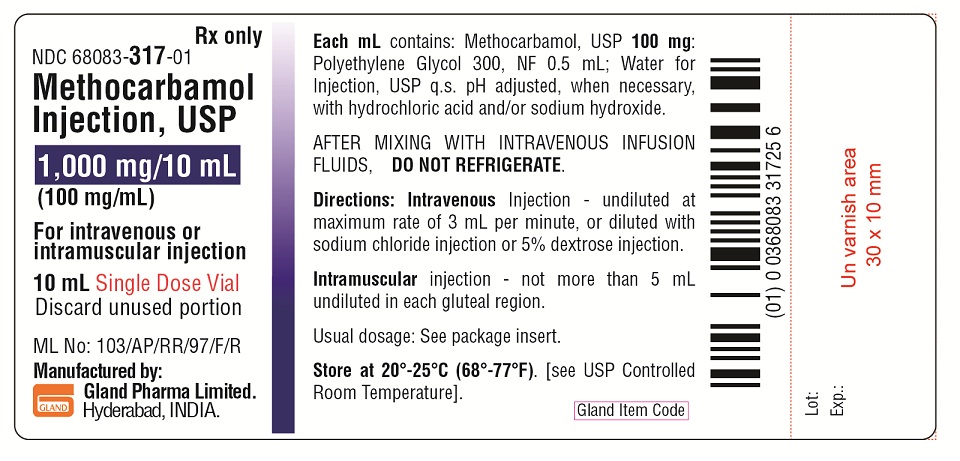
Each mL contains: methocarbamol, USP 100 mg, polyethylene glycol 300, NF 0.5 mL, Water for Injection, USP q.s. The pH is adjusted, when necessary, with hydrochloric acid and/or sodium hydroxide. The chemical name of methocarbamol is 3-(2-methoxyphenoxy)-1,2-propanediol 1-carbamate and has the empirical formula of C11H15NO5. Its molecular weight is 241.24. The structural formula is shown below:
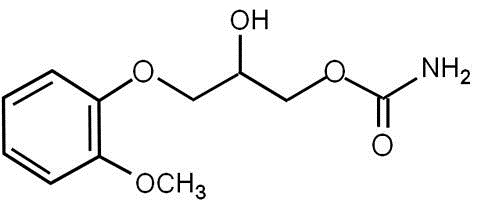
Methocarbamol is a white powder, sparingly soluble in water and chloroform, soluble in alcohol (only with heating) and propylene glycol, and insoluble in benzene and n-hexane.
Methocarbamol Injection, USP has a pH between 3.5 and 6.0.
AFTER MIXING WITH INTRAVENOUS INFUSION FLUIDS, DO NOT REFRIGERATE.
CLINICAL PHARMACOLOGY
The mechanism of action of methocarbamol in humans has not been established, but may be due to general CNS depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
Pharmacokinetics
In healthy volunteers, the plasma clearance of methocarbamol ranges between 0.20 and 0.80 L/h/kg, the mean plasma elimination half-life ranges between 1 and 2 hours, and the plasma protein binding ranges between 46% and 50%.
Methocarbamol is metabolized via dealkylation and hydroxylation. Conjugation of methocarbamol also is likely. Essentially all methocarbamol metabolites are eliminated in the urine. Small amounts of unchanged methocarbamol also are excreted in the urine.
SPECIAL POPULATIONS
Elderly
The mean (±SD) elimination half-life of methocarbamol in elderly healthy volunteers (mean (±SD) age, 69 (±4) years) was slightly prolonged compared to a younger (mean (±SD) age, 53.3 (±8.8) years), healthy population (1.5 (±0.4) hours versus 1.1 (±0.27) hours, respectively). The fraction of bound methocarbamol was slightly decreased in the elderly versus younger volunteers (41% to 43% versus 46% to 50%, respectively).
Renally Impaired
The clearance of methocarbamol in 8 renally-impaired patients on maintenance hemodialysis was reduced about 40% compared to 17 normal subjects, although the mean (±SD) elimination half-life in these two groups was similar (1.2 (±0.6) versus 1.1 (±0.3) hours, respectively).
Hepatically Impaired
In 8 patients with cirrhosis secondary to alcohol abuse, the mean total clearance of methocarbamol was reduced approximately 70% compared to that obtained in 8 age- and weight-matched normal subjects. The mean (±SD) elimination half-life in the cirrhotic patients and the normal subjects was 3.38 (±1.62) hours and 1.11 (±0.27) hours respectively. The percent of methocarbamol bound to plasma proteins was decreased to approximately 40% to 45% compared to 46% to 50% in the normal subjects.
INDICATIONS AND USAGE
The injectable form of methocarbamol is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
CONTRAINDICATIONS
Methocarbamol Injection should not be administered to patients with known or suspected renal pathology. This caution is necessary because of the presence of polyethylene glycol 300 in the vehicle.
A much larger amount of polyethylene glycol 300 than is present in recommended doses of Methocarbamol Injection is known to have increased pre-existing acidosis and urea retention in patients with renal impairment. Although the amount present in this preparation is well within the limits of safety, caution dictates this contraindication.
Methocarbamol Injection is contraindicated in patients hypersensitive to methocarbamol or to any of the injection components.
WARNINGS
Since methocarbamol may possess a general CNS depressant effect, patients receiving Methocarbamol Injection should be cautioned about combined effects with alcohol and other CNS depressants.
Safe use of Methocarbamol Injection has not been established with regard to possible adverse effects upon fetal development. There have been very rare reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, Methocarbamol Injection should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see PRECAUTIONS, Pregnancy).
Use in Activities Requiring Mental Alertness
Methocarbamol may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that methocarbamol therapy does not adversely affect their ability to engage in such activities.
PRECAUTIONS
General
As with other agents administered either intravenously or intramuscularly, careful supervision of dose and rate of injection should be observed. Rate of injection should not exceed 3 mL per minute–i.e., one 10 mL vial in approximately three minutes. Since Methocarbamol Injection is hypertonic, vascular extravasation must be avoided. A recumbent position will reduce the likelihood of side reactions.
Blood aspirated into the syringe does not mix with the hypertonic solution. This phenomenon occurs with many other intravenous preparations. The blood may be injected with the methocarbamol, or the injection may be stopped when the plunger reaches the blood, whichever the physician prefers.
The total dosage should not exceed 30 mL (three vials) a day for more than three consecutive days except in the treatment of tetanus.
Caution should be observed in using the injectable form in patients with suspected or known seizure disorders.
Information for Patients
Patients should be cautioned that methocarbamol may cause drowsiness or dizziness, which may impair their ability to operate motor vehicles or machinery.
Because methocarbamol may possess a general CNS-depressant effect, patients should be cautioned about combined effects with alcohol and other CNS depressants.
Drug Interactions
See WARNINGS and PRECAUTIONS for interaction with CNS drugs and alcohol.
Methocarbamol may inhibit the effect of pyridostigmine bromide. Therefore, methocarbamol should be used with caution in patients with myasthenia gravis receiving anticholinesterase agents.
Drug/Laboratory Test Interactions
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) using nitrosonaphthol reagent and in screening tests for urinary vanillylmandelic acid (VMA) using the Gitlow method.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of methocarbamol have not been performed. No studies have been conducted to assess the effect of methocarbamol on mutagenesis or its potential to impair fertility.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with methocarbamol. It is also not known whether methocarbamol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methocarbamol Injection should be given to a pregnant woman only if clearly needed.
Safe use of Methocarbamol Injection has not been established with regard to possible adverse effects upon fetal development. There have been reports of fetal and congenital abnormalities following in utero exposure to methocarbamol. Therefore, Methocarbamol Injection should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards (see WARNINGS).
Nursing Mothers
Methocarbamol and/or its metabolites are excreted in the milk of dogs; however, it is not known whether methocarbamol or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Methocarbamol Injection is administered to a nursing woman.
ADVERSE REACTIONS
The following adverse reactions have been reported coincident with the administration of methocarbamol. Some events may have been due to an overly rapid rate of intravenous injection.
Body as a whole: Anaphylactic reaction, angioneurotic edema, fever, headache
Cardiovascular system: Bradycardia, flushing, hypotension, syncope, thrombophlebitis
In most cases of syncope there was spontaneous recovery. In others, epinephrine, injectable steroids, and/or injectable antihistamines were employed to hasten recovery.
Digestive system: Dyspepsia, jaundice (including cholestatic jaundice), nausea and vomiting
Hemic and lymphatic system: Leukopenia
Immune system: Hypersensitivity reactions
Nervous system: Amnesia, confusion, diplopia, dizziness or light-headedness, drowsiness, insomnia, mild muscular incoordination, nystagmus, sedation, seizures (including grand mal), vertigo
The onset of convulsive seizures during intravenous administration of methocarbamol has been reported in patients with seizure disorders. The psychic trauma of the procedure may have been a contributing factor. Although several observers have reported success in terminating epileptiform seizures with Methocarbamol Injection, its administration to patients with epilepsy is not recommended (see PRECAUTIONS, General).
Skin and special senses: Blurred vision, conjunctivitis, nasal congestion, metallic taste, pruritus, rash, urticaria
Other: Pain and sloughing at the site of injection
To report SUSPECTED ADVERSE REACTIONS, contact Gland Pharma at 864-879-9994 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Limited information is available on the acute toxicity of methocarbamol. Overdose of methocarbamol is frequently in conjunction with alcohol or other CNS depressants and includes the following symptoms: nausea, drowsiness, blurred vision, hypotension, seizures, and coma. In post-marketing experience deaths have been reported with an overdose of methocarbamol alone or in the presence of other CNS depressants, alcohol or psychotropic drugs.
Treatment
Management of overdose includes symptomatic and supportive treatment. Supportive measures include maintenance of an adequate airway, monitoring urinary output and vital signs, and administration of intravenous fluids if necessary. The usefulness of hemodialysis in managing overdose is unknown.
DOSAGE AND ADMINISTRATION
For Intravenous and Intramuscular Use Only. Total adult dosage should not exceed 30 mL (3 vials) a day for more than 3 consecutive days except in the treatment of tetanus. If the condition persists, a like course may be repeated after a drug-free interval of 48 hours. Dosage and frequency of injection should be based on the severity of the condition being treated and therapeutic response noted.
For the relief of symptoms of moderate degree, one dose of 1 gram (one 10 mL vial) may be adequate. Ordinarily this injection need not be repeated, as the administration of the oral form will usually sustain the relief initiated by the injection. For the severest cases or in postoperative conditions in which oral administration is not feasible, additional doses of 1 gram may be repeated every 8 hours up to a maximum of 3 grams per day for no more than 3 consecutive days.
Directions for Intravenous Use
Methocarbamol Injection may be administered undiluted directly into the vein at a maximum rate of three mL per minute. It may also be added to an intravenous drip of Sodium Chloride Injection (Sterile Isotonic Sodium Chloride Solution for Parenteral Use) or five percent Dextrose Injection (Sterile 5 percent Dextrose Solution); one vial given as a single dose should not be diluted to more than 250 mL for I.V. infusion. AFTER MIXING WITH INTRAVENOUS INFUSION FLUIDS, DO NOT REFRIGERATE. Care should be exercised to avoid vascular extravasation of this hypertonic solution, which may result in thrombophlebitis. It is preferable that the patient be in a recumbent position during and for at least 10 to 15 minutes following the injection.
Directions for Intramuscular Use
When the intramuscular route is indicated, not more than five mL (one-half vial) should be injected into each gluteal region. The injections may be repeated at eight hour intervals, if necessary. When satisfactory relief of symptoms is achieved, it can usually be maintained with tablets.
Not Recommended for Subcutaneous Administration.
Special Directions for Use in Tetanus
There is clinical evidence which suggests that methocarbamol may have a beneficial effect in the control of the neuromuscular manifestations of tetanus. It does not, however, replace the usual procedure of debridement, tetanus antitoxin, penicillin, tracheotomy, attention to fluid balance, and supportive care. Methocarbamol Injection should be added to the regimen as soon as possible.
For Adults: Inject one or two vials directly into the tubing of the previously inserted indwelling needle. An additional 10 mL or 20 mL may be added to the infusion bottle so that a total of up to 30 mL (three vials) is given as the initial dose (see PRECAUTIONS). This procedure should be repeated every six hours until conditions allow for the insertion of a nasogastric tube. Crushed methocarbamol tablets suspended in water or saline may then be given through this tube. Total daily oral doses up to 24 grams may be required as judged by patient response.
For Pediatric Patients: A minimum initial dose of 15 mg/kg or 500 mg/m2 is recommended. This dosage may be repeated every six hours, if required. The total dose should not exceed 1.8 g/m2 for 3 consecutive days. The maintenance dosage may be given by injection into tubing or by I.V. infusion with an appropriate quantity of fluid. See Directions for Intravenous Use.
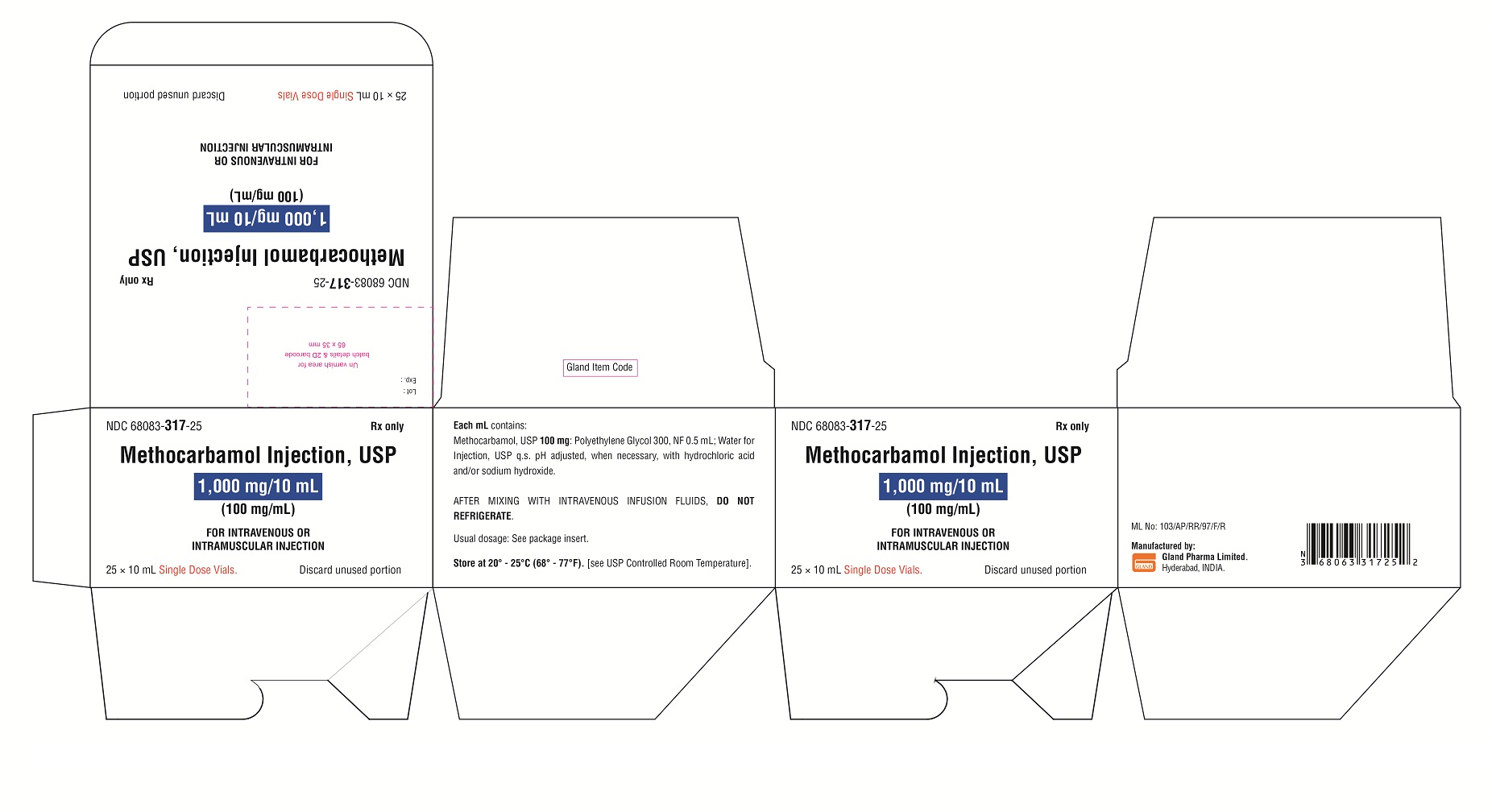
HOW SUPPLIED
Methocarbamol Injection, USP, is a clear colorless solution supplied as follows:
NDC Methocarbamol Injection, USP (100 mg/mL) Package Factor
68083-317-25 1,000 mg/10 mL Single-Dose Vial 25 vials per carton
Storage Conditions
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
AFTER MIXING WITH INTRAVENOUS INFUSION FLUIDS, DO NOT REFRIGERATE.
Discard unused portion.
Sterile, Nonpyrogenic, Preservative-free.
The container closure is not made with natural rubber latex.
Manufactured by:
Gland Pharma Limited
D.P.Pally, Dundigal Post
Hyderabad - 500 043, INDIA
Revised: July 2020