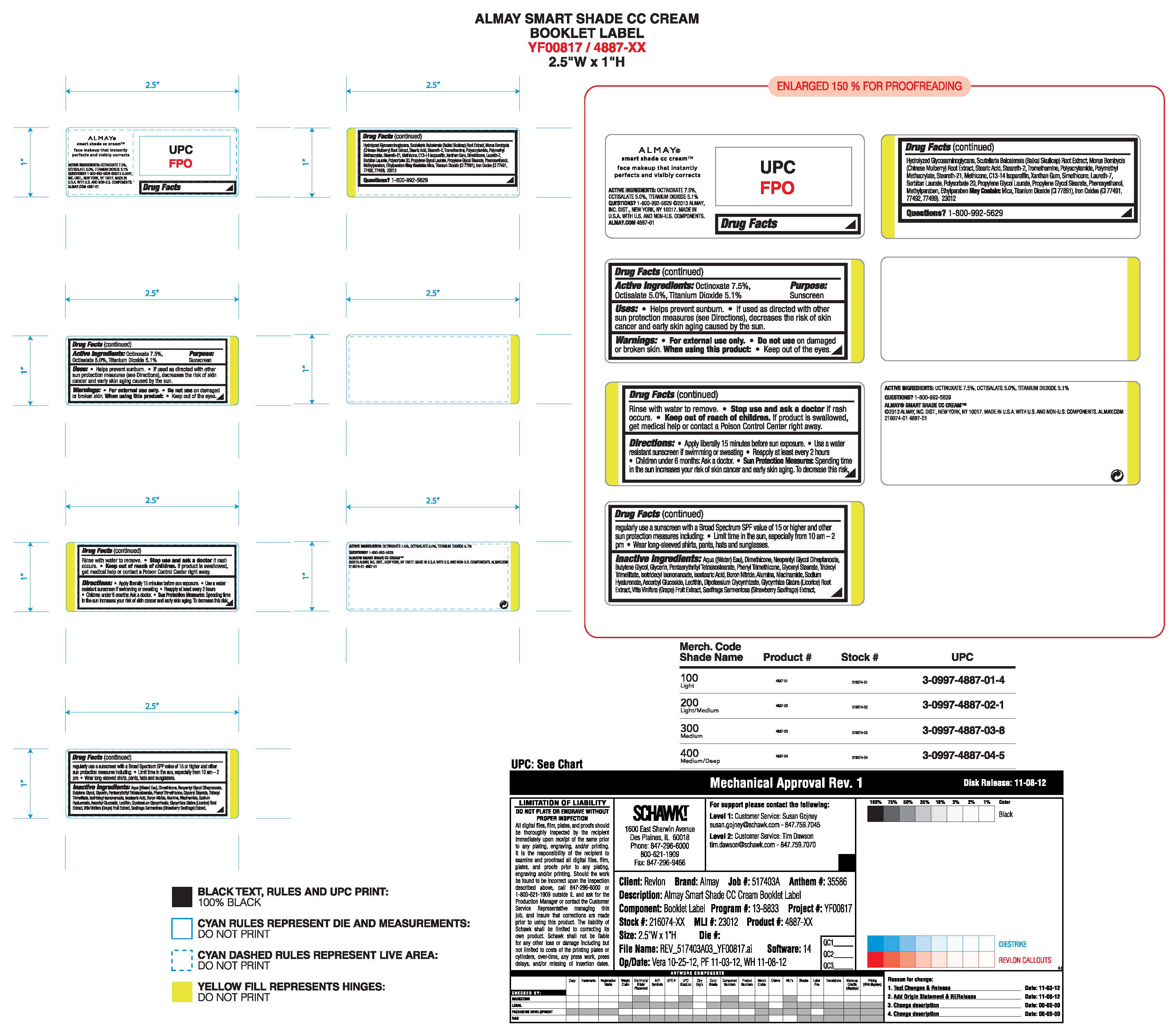
Uses:
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings:
- For external use only
- Do notuse on damaged or broken skin
- When using this product: Keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if a rash occurs
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control center right away
Directions:
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor.
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 am - 2 pm
- Wear long-sleeved shirts, pants, hats and sunglasses.
Inactive Ingredients:
AQUA ((WATER) EAU),DIMETHICONE,NEOPENTYL GLYCOL DIHEPTANOATE,BUTYLENE GLYCOL,GLYCERIN,PENTAERYTHRITYL TETRAISOSTEARATE,PHENYL TRIMETHICONE,GLYCERYL STEARATE,TRIDECYL TRIMELLITATE,ISOTRIDECYL ISONONANOATE,ISOSTEARIC ACID,BORON NITRIDE,ALUMINA,PALMITOYL TRIPEPTIDE-5,ACETYL HEXAPEPTIDE-8,GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT,VITIS VINIFERA (GRAPE) FRUIT EXTRACT,SAXIFRAGA SARMENTOSA (STRAWBERRY SAXIFRAGE) EXTRACT,SODIUM HYALURONATE,HYDROLYZED GLYCOSAMINOGLYCANS,SCUTELLARIA BAICALENSIS (BAIKAL SKULLCAP) ROOT EXTRACT,MORUS BOMBYCIS(CHINES MULBERRY) ROOT EXTRACT,ASCORBYL GLUCOSIDE,NIACINAMIDE,DIPOTASSIUM GLYCYRRHIZATE,C13-14 ISOPARAFFIN,XANTHAN GUM,SIMETHICONE,LAURETH-7,LECITHIN,SORBITAN LAURATE,POLYSORBATE 20,PROPYLENE GLYCOL LAURATE,PROPYLENE GLYCOL STEARATE,METHICONE,STEARIC ACID,STEARETH-2,TROMETHAMINE,POLYACRYLAMIDE,POLYMETHYL METHACRYLATE,STEARETH-21,PHENOXYETHANOL,METHYLPARABEN,ETHYLPARABEN
May Contain Mica, Titanium Dioxide (Ci 77891), Iron Oxides (CI 77491, 77492, 77499)