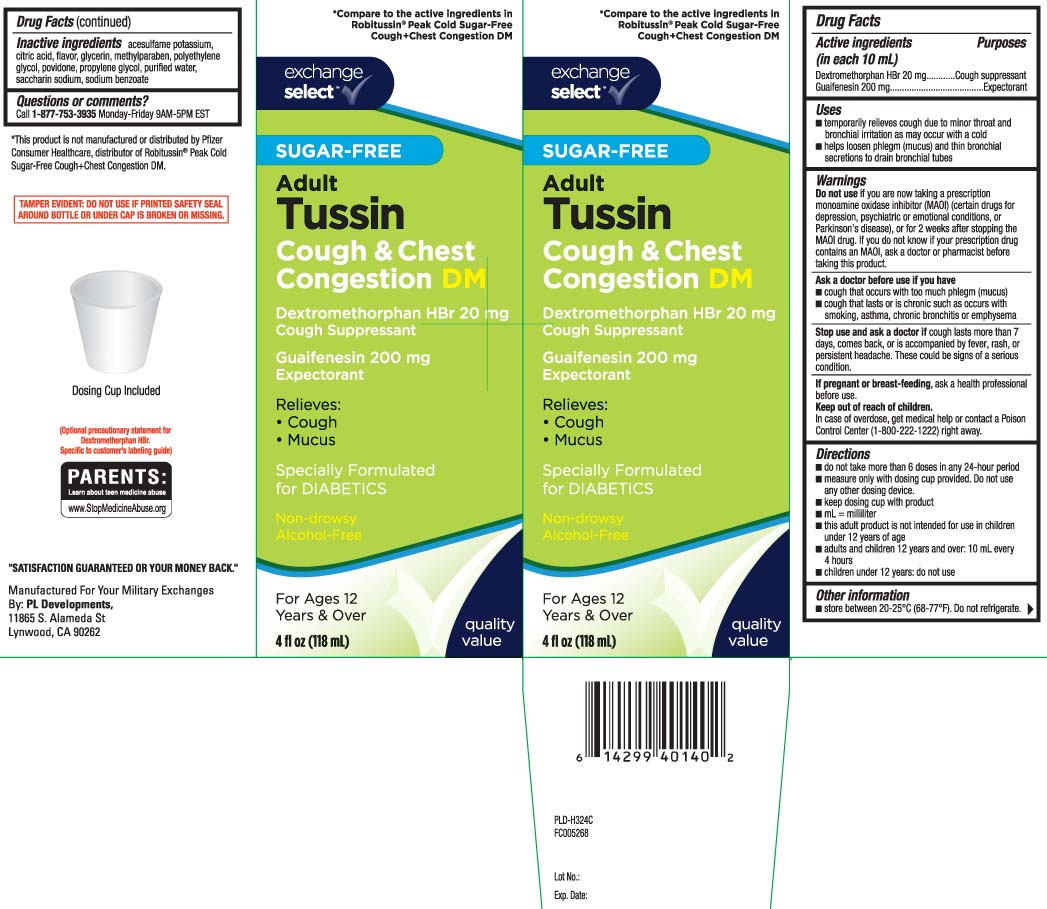
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided. Do not use any other dosing device.
- keep dosing cup with product
- mL = milliliter
- this adult product is not intended for use in children under 12 years of age
- adults and children 12 years and over: 10 mL every 4 hours
- children under 12 years: do not use
Inactive ingredients
acesulfame potassium, citric acid, flavors, glycerin, methylparaben, polyethylene glycol, povidone, propylene glycol, purified water, saccharin sodium, sodium benzoate
Principal Display Panel
*Compare to active ingredients in Robitussin® Peak Cold Sugar-Free Cough & Chest Congestion DM
SUGAR-FREE
Adult
Tussin
Cough & Chest Congestion DM
Dextromethorphan HBr 20 mg
Cough suppressant
Guaifenesin 200 mg
Expectorant
Relieves:
- Cough
- Chest congestion
Specially Formulated for DIABETICS
Non-drowsy
Alcohol-Free
For Ages 12 Years & over
Dosing Cup Included
FL OZ (mL)
*This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of Robitussin® Peak Cold Sugar Free Cough & Chest COngestion DM
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL AROUND BOTTLE OR UNDER CAP IS BROKEN OR MISSING.
Manufactured for your military exchanges
By: PL developments
11865 S Alameda St
Lynowood, CA 90262