VERMAFUGE -AG
LYCOPODIUM- nux vomica,arsenicum album,cinchona officinalis,capsicum annuum,nitric acid,mercuriuscorrosivus,belladonna,cajuputum,lycopodium clavatum,phosphorus,phytolacca decandra,rhamnus purshiana,fragaria vesca liquid
White Manufacturing Inc. DBA Micro-West
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS
Arsenicum alb 12X
Belladonna 12X
Cajuputum 4X
Capsicum 3X
Cinchona 12X
Fragaria 12X
Lycopodium 12X
Merc corros 12X
Nitricum ac 12X
Nux vom 12X
Phos 12X
Phytolacca 6X
Rhamnus pursh 3X
PURPOSE
FOR THE TEMPORARY RELIEF OF DYSENTERY, FLATULENCE, AND COLIC
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
WARNING
WARNINGS: STOP USE AND CALL A DOCTOR if symptoms persist or worsen. IF PREGNANT OR BREAST FEEDING, consult a healthcare professional before use. CONTAINS ALCOHOL: in case of accidental overdose, consult a poison control center immediately.
OTHER SAFETY INFORMATION
OTHER INFORMATION: Tamper resistant for your protection. Use only if safety seal is intact.
DIRECTIONS
DIRECTIONS: Take 6 drops orally, 4 times a day. For ages 2 to adult
INDICATIONS
INDICATIONS: FOR THE TEMPORARY RELIEF OF DYSENTARY, FLATULENCE, AND COLIC
INACTIVE INGREDIENT
Alcohol 20% v/v
Purified water
MANUFACTURE
MANUFACTURED FOR
MICRO-WEST
P.O. BOX 950
DOUGLAS, WY 82633
1-307-358-5066
LABEL
NDC 61657-1026-1
MICRO-WEST
Homeopathic
VERMAFUGE-AG
Product #: 1026
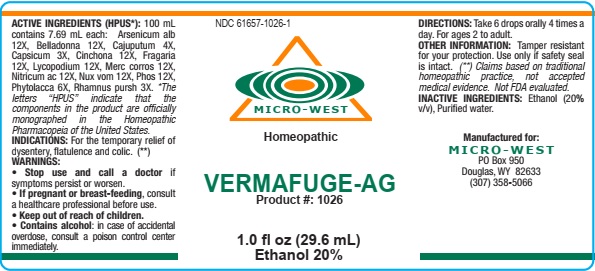
1.0 fl oz (29.6 mL)
Ethanol 20%
White Manufacturing Inc. DBA Micro-West
