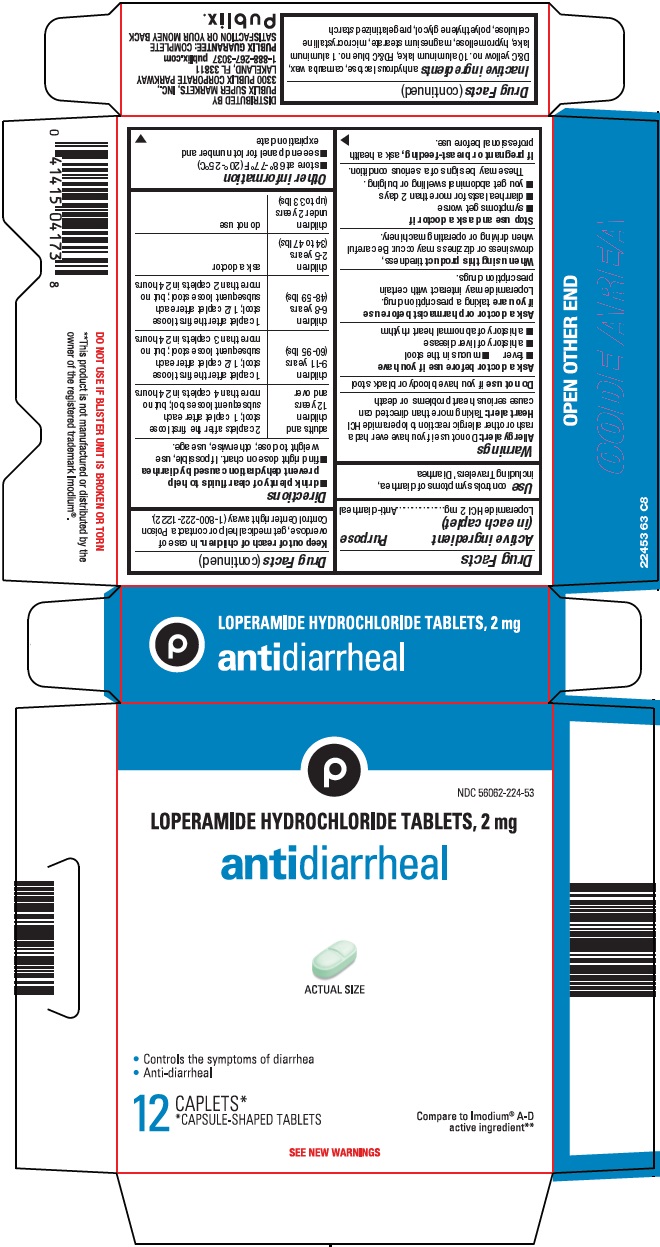
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
Heart alert: Taking more than directed can cause serious heart problems or death
Ask a doctor before use if you have
- •
- fever
- •
- mucus in the stool
- •
- a history of liver disease
- •
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product
tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Directions
- •
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- •
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
|
adults and children 12 years and over |
2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
|
children 9-11 years (60-95 lbs) |
1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
|
children 6-8 years (48-59 lbs) |
1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
|
children 2-5 years (34 to 47 lbs) |
ask a doctor |
|
children under 2 years (up to 33 lbs) |
do not use |