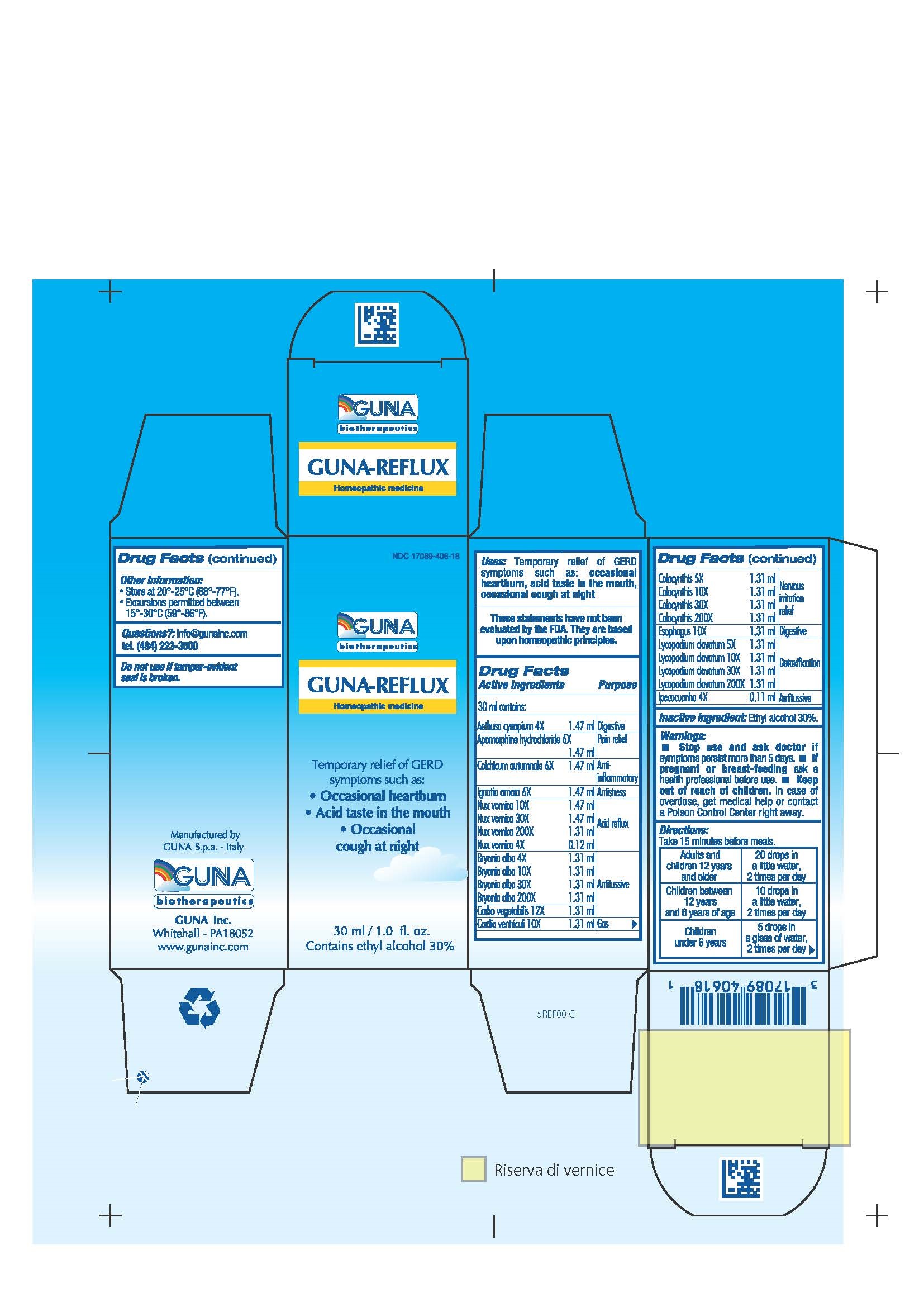
ACTIVE INGREDIENTS/PURPOSE
AETHUSA CYNAPIUM 4X DIGESTIVE
APOMORPHINE HYDROCHLORIDE 6X PAIN RELIEF
BRYONIA ALBA 4X, 10X, 30X, 200X ANTITUSSIVE
CARBO VEGETALIS 12X ANTITUSSIVE
CARDIA VENTRICULI 10X GAS
COLCHICUM AUTUMNALE 6X ANTI-INFLAMMATORY
COLOCYNTHIS 5X, 10X, 30X, 200X NERVOUS IRRITATION RELIEF
ESOPHAGUS 10X DIGESTIVE
IGNATIA AMARA 6X ANTISTRESS
IPECACUANHA 4X ANTITUSSIVE
LYCOPODIUM CLAVATUM 5X, 10X, 30X, 200X DETOXIFICATION
NUX VOMICA 4X, 10X, 30X, 200X ACID REFLUX
USES
Temporary relief of GERD symptoms such as:
- Occasional heartburn
- Acir taste in the mouth
- Occasional cough at night
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Contains ethyl alcohol 30%