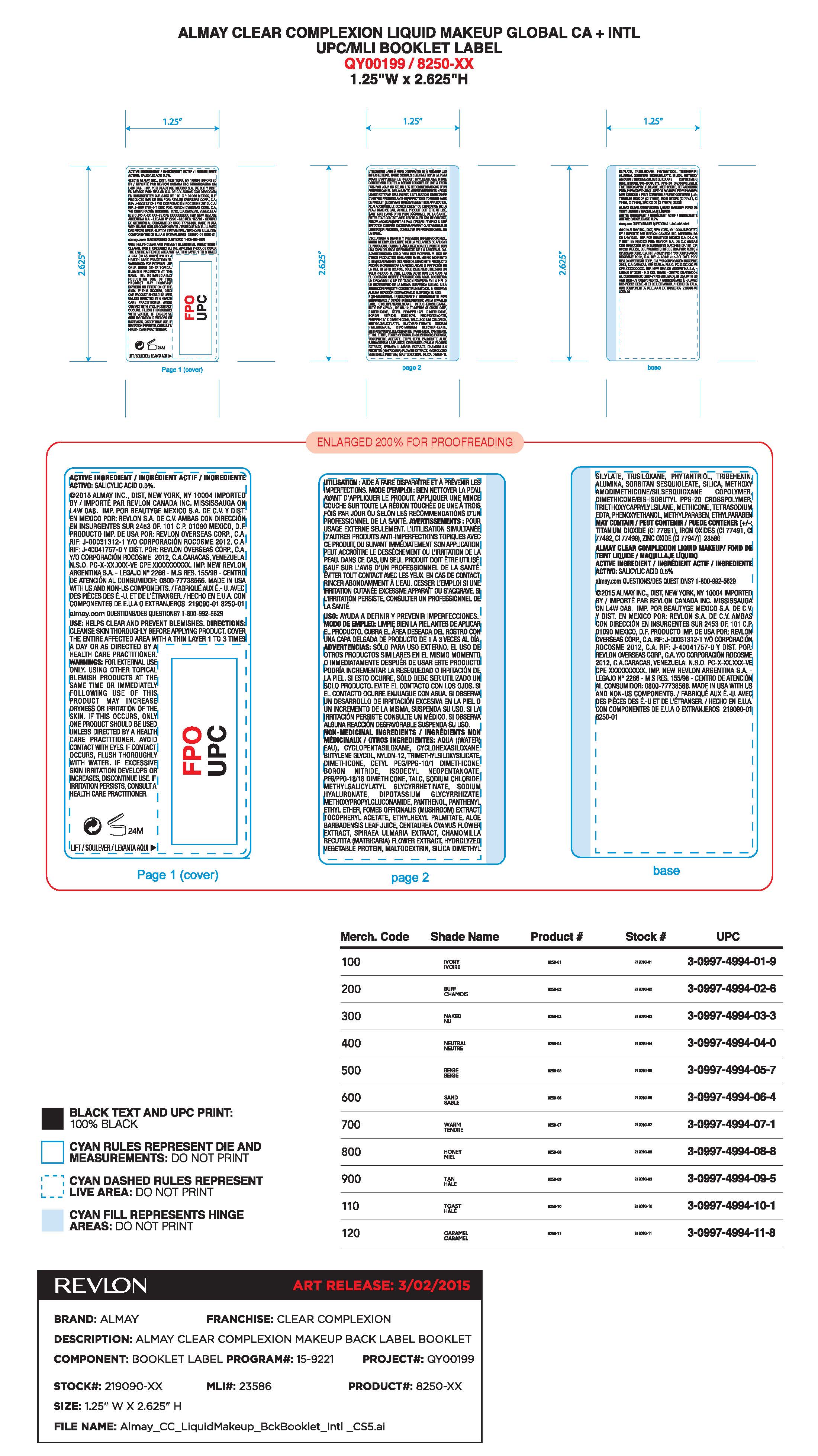
Warnings:
•FOR EXTERNAL USE ONLY. USING OTHER TOPICAL ACNE MEDICATIONS AT THE SAME TIME OR
IMMEDIATELY FOLLOWING USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE
SKIN. IF THIS OCCURS, ONLY ONE MEDICATION SHOULD BE USED UNLESS DIRECTED BY A DOCTOR.ter right away
Directions
Cleanse skin thoroughly before applying product. Cover the enitre affected area with a thin layer 1 to 3 times a day or as directed by health care practitioner.
Inactive Ingredients
AQUA ((WATER) EAU), CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, BUTYLENE GLYCOL, NYLON-12, TRIMETHYLSILOXYSILICATE, DIMETHICONE, CETYL PEG/PPG-10/1 DIMETHICONE, BORON NITRIDE, ISODECYL NEOPENTANOATE, PEG/PPG-18/18 DIMETHICONE, TALC, SODIUM CHLORIDE, METHYLSALICYLATYL GLYCYRRHETINATE, SODIUM HYALURONATE, DIPOTASSIUM GLYCYRRHIZATE, METHOXYPROPYLGLUCONAMIDE, PANTHENOL, PANTHENYL ETHYL ETHER, FOMES OFFICINALIS (MUSHROOM) EXTRACT, TOCOPHERYL ACETATE, ETHYLHEXYL PALMITATE, ALOE BARBADENSIS LEAF JUICE, CENTAUREA CYANUS FLOWER EXTRACT, SPIRAEA ULMARIA EXTRACT, CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT, HYDROLYZED VEGETABLE PROTEIN, MALTODEXTRIN, SILICA DIMETHYL SILYATE, TRISILOXANE, PHYTANTRIOL, TRIBEHENIN, ALUMINA, SORBITAN SESQUIOLEATE, SILICA, METHOXY AMODIMETHICONE/SISESQUIOXANE COPOLYMER, DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER, TRIETHOXYCAPRYLYSILANE, METHICONE, TETRASODIUM EDTA, PHENOXYETHANOL, METHYLPARABEN, ETHYLPARABEN