Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
Heart alert: Taking more than directed can cause serious heart problems or death
Ask a doctor before use if you have
- fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
Stop use and ask a doctor if
- symptoms get worse
- diarrhea lasts for more than 2 days
- you get abdominal swelling or bulging. These may be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea.
- not for use in children under 12 years of age
- adults and children 12 years and over: 2 capsules after the first loose stool; 1 capsule after each subsequent loose stool; but no more than 4 capsules in 24 hours
Other information
- store at 20°-25°C (68°-77°F). Protect from Light.
- avoid excessive heat above 40°C (104°F)
- do not use if carton or blister unit is open or torn
- see side panel for lot number and expiration date
Inactive ingredients
butylated hydroxyanisole, edible ink, FD&C Blue #1, gelatin, glycerin, glyceryl caprylate, polyoxyl 40 hydrogenated castor oil, purified water
Questions or comments?
call toll free 1-888-235-2466
DIST. MEIJER DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.com
†This product is not manufactured or distributed by Johnson & Johnson, owner of the registered trademark Imodium ® A-D.
THIS PRODUCT IS PACKAGED IN A CHILD-RESISTANT AND TAMPER EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
L0000567
R0322
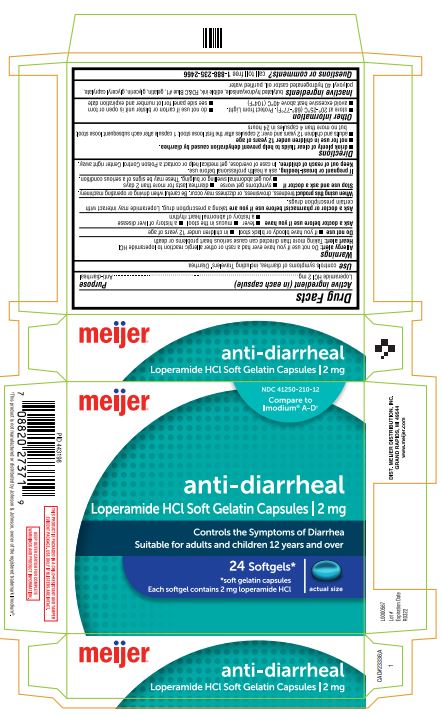
Principal Display Panel
meijer
NDC 41250-210-12
Compare to Imodium® A-D†
anti-diarrheal
Loperamide HCl Soft Gelatin Capsules | 2 mg
Controls the Symptoms of Diarrhea
Suitable for adults and children 12 years and over
24 Softgels*
*soft gelatin capsules
Each softgel contains 2 mg loperamide HCl
actual size