Uses
- relieves redness of the eye due to minor eye irritations
- for use as a protectant against further irritation or to relieve dryness of the eye
- for the temporarily relief of burning and irritation due to dryness of the eye
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- overuse may produce increased redness of the eye
- remove contact lenses before using
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate
Dist by Medtech Products Inc., Tarrytown, NY 10591, a Prestige Brands Company
Distributed by Convenience Valet® Melrose Park, IL 60160
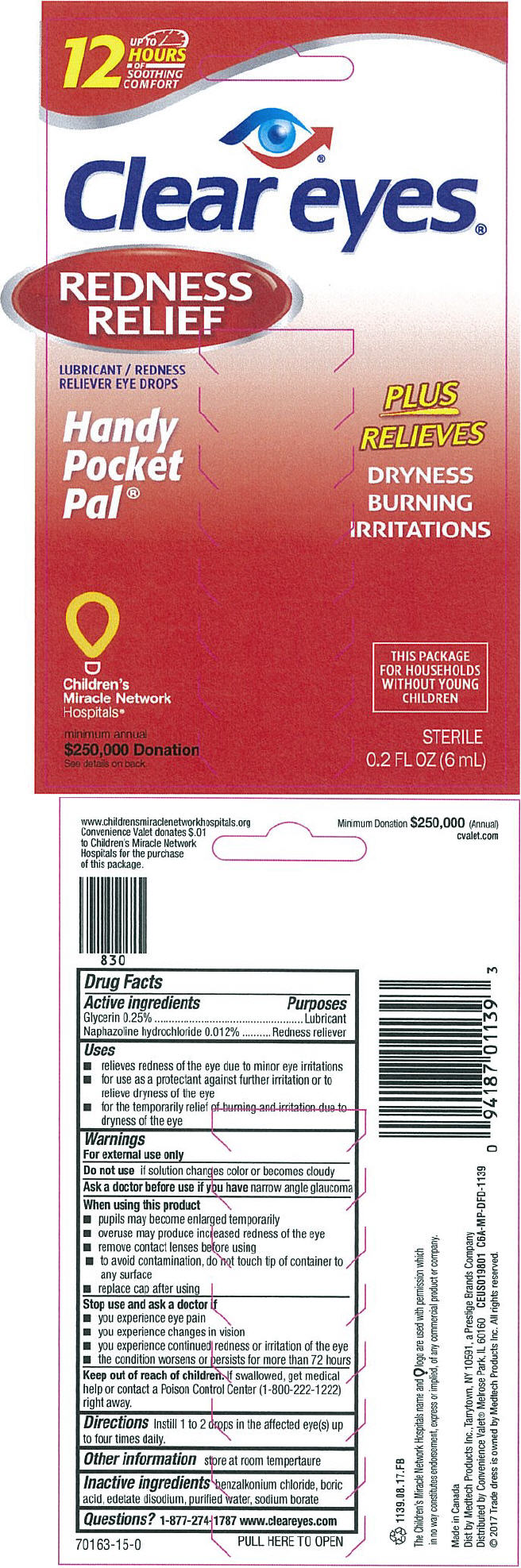
PRINCIPAL DISPLAY PANEL - 6 mL Bottle Box
UP TO
12
HOURS
OF
SOOTHING
COMFORT
Clear eyes®
REDNESS
RELIEF
LUBRICANT / REDNESS
RELIEVER EYE DROPS
Handy
Pocket
Pal®
PLUS
RELIEVES
DRYNESS
BURNING
IRRITATIONS
Children's
Miracle Network
Hospitals®
minimum annual
$250,000 Donation
See details on back
THIS PACKAGE
FOR HOUSEHOLDS
WITHOUT YOUNG
CHILDREN
STERILE
0.2 FL OZ (6 mL)