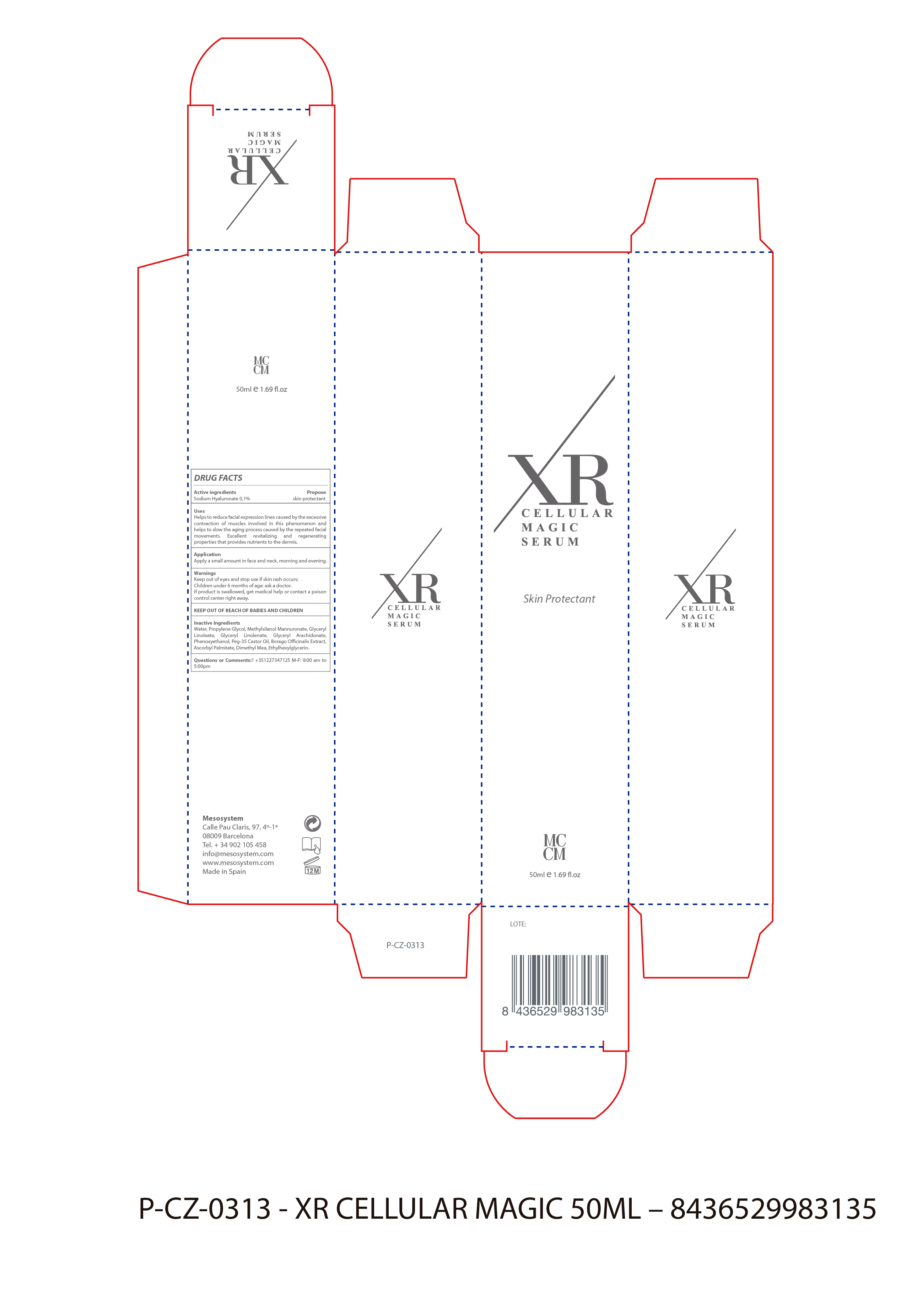
WARNINGS
- For external use only
- Stop use and ask a doctor if skin rash occur.s
- Avoid the product from entering your eyes
- Children under 6 months Ask a Doctor
USES
- Helps to reduce facial expression lines caused by the excessive contraction of muscles envolved in this phenomenos and
- helps to slow the aging process caused by the repeated facil movements. Excellent revitalizing and regenerating properties that provides nutrient to the dermis
- Keep out of the reach of chuldren.
- In case of overdose get medical help or Contact a Poison Control Center right away.
ACTIVE INGREDIENTS
Active Ingredients Purpose
Sodium Hyaluronate 0.1%....................................Skin Protectant