GUAIFENESIN400MG TABLETS
MarlexPharmaceuticals, Inc.
----------
Drug Fact
Active ingredient (in each tablet)
Guaifenesin 400mg
Purpose
Expectorant
Guaifenesin 400 mg Tablets
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageway of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- Persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
cough last more than 7 days, come back or is accompanied by fever, rash, or persistent headache. There could be signs of a serious illness.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
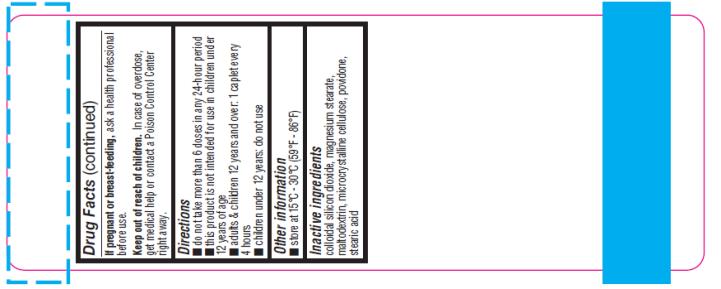
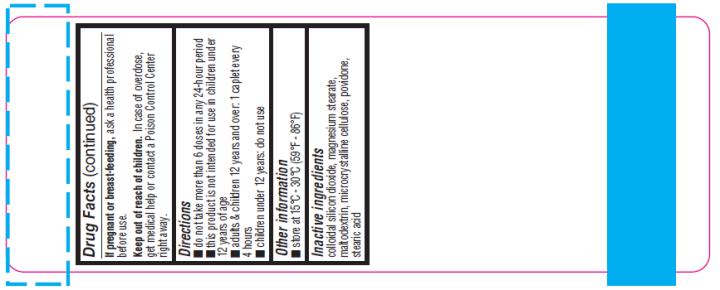
If pregnant or breast- feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions:
- Do not take more than 6 doses in any 24-hour period.
- This product is not intended for use in children under 12 years of age
- Adults & children 12 years and over: 1 to 2 tablets every 4 hours
- Children under 12 years: do not use
Other Information:
- Store at 15°C-30°C (59°F-86°F)
Inactive Ingredients:
Colloidal silicon dioxide, FD&C RED #40(AI-lake), magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, stearic acid
Manufactured for & Distributed by:
Marlex Pharmaceuticals, Inc.
New Castle, DE 19720
Rev: 02/19TCL
PRINCIPAL DISPLAY PANEL
NDC 10135-0682-30
Guaifenesin
400 mg
30 CAPLETS

PRINCIPAL DISPLAY PANEL
NDC 10135-0682-60
Guaifenesin
400 mg
60 CAPLETS