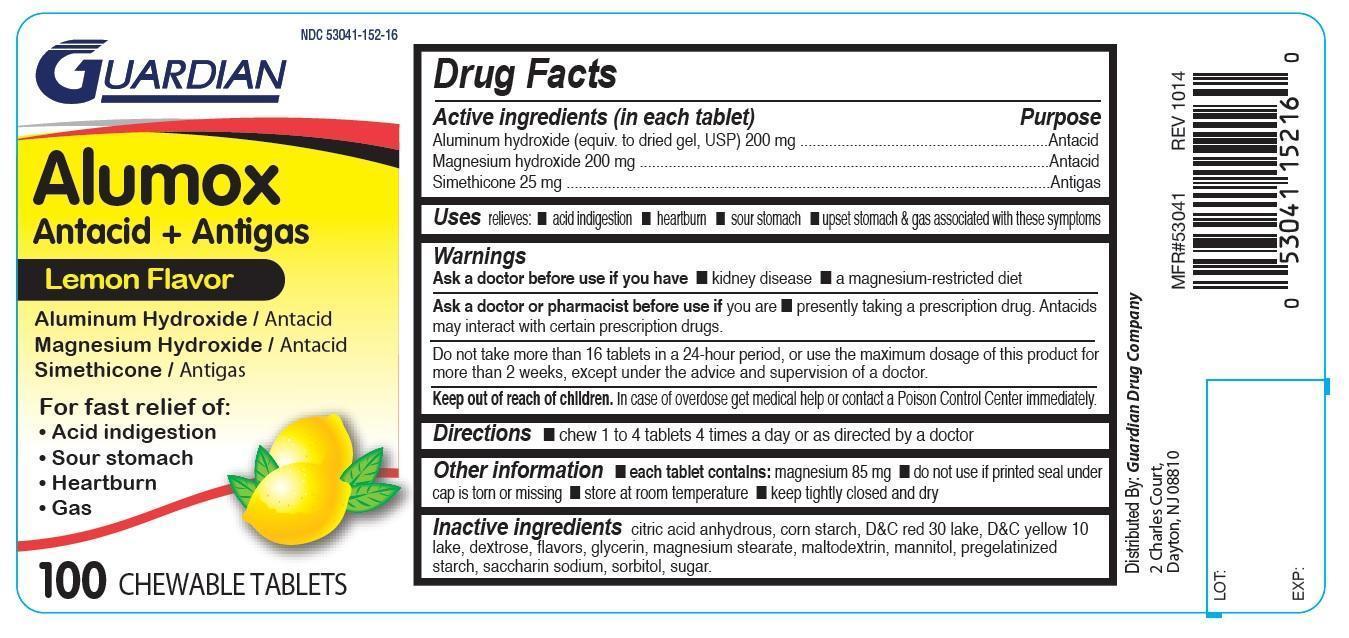
ALUMOX- aluminum hydroxide, magnesium hydroxide, simethicone tablet, chewable
Guardian Drug Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients (in each tablet)
Aluminum hydroxide (equiv. to dried gel, USP) 200 mg
Magnesium hydroxide 200 mg
Simethicone 25 mg
Purpose
Antacid
Antacid
Antigas
Uses
relieves:
acid indigestion
heartburn
sour stomach
upset stomach & gas associated with these symptoms
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
Ask a doctor or pharmacist before use if you are
- presently taking a prescription drug. Antacids may interact with certain certain prescription drugs.
Do not take
more than 16 tablets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
keep out of reach of children. In case of overdose get medical help or contact a poison control center immediately.
Directions
- chew 1 to 4 tablets 4 times a day or as directed by a doctor.
Other information
- each tablet contains: magnesium 85 mg
- do not use if seal under cap is torn or missing
- store at room temperature
- keep tightly closed and dry
Inactive ingredients
citric acid anhydrous, corn starch, D&C red 30 lake, D&C yellow 10 lake, dextrose, flavor, glycerin, magnesium stearate, maltodextrin, mannitol, pregelatinized starch, saccharin sodium, sorbitol, sugar
PDP
Alumox
Antacid+Antigas
Lemon Flavor
Aluminum Hydroxide / Antacid
Magnesium Hydroxide / Antacid
Simethicone / Antigas
FOR FAST RELIEF OF:
Acid indigestion
Sour stomach
Heartburn
Gas
100 Chewable Tablets