Warnings
Ask a doctor or pharmacist before use if you are now taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- chew 2 to 4 tablets four times a day or as directed by a doctor.
- take after meals and at bedtime or as needed
- for best results follow by a half glass of water or other liquid
- do not swallow whole
Other information
- each tablet contains :
magnesium 50 mg and sodium 32 mg - store at 20° -25°C.
- See end flap for expiration date and lot number.
Inactive ingredients
alginic acid, calcium stearate, flavor, mannitol, sodium bicarbonate, starch, sucrose, water, FD&C Yellow # 5 (tartrazine), FD&C Yellow # 6, lactose, dextrose, talcum, sucralose
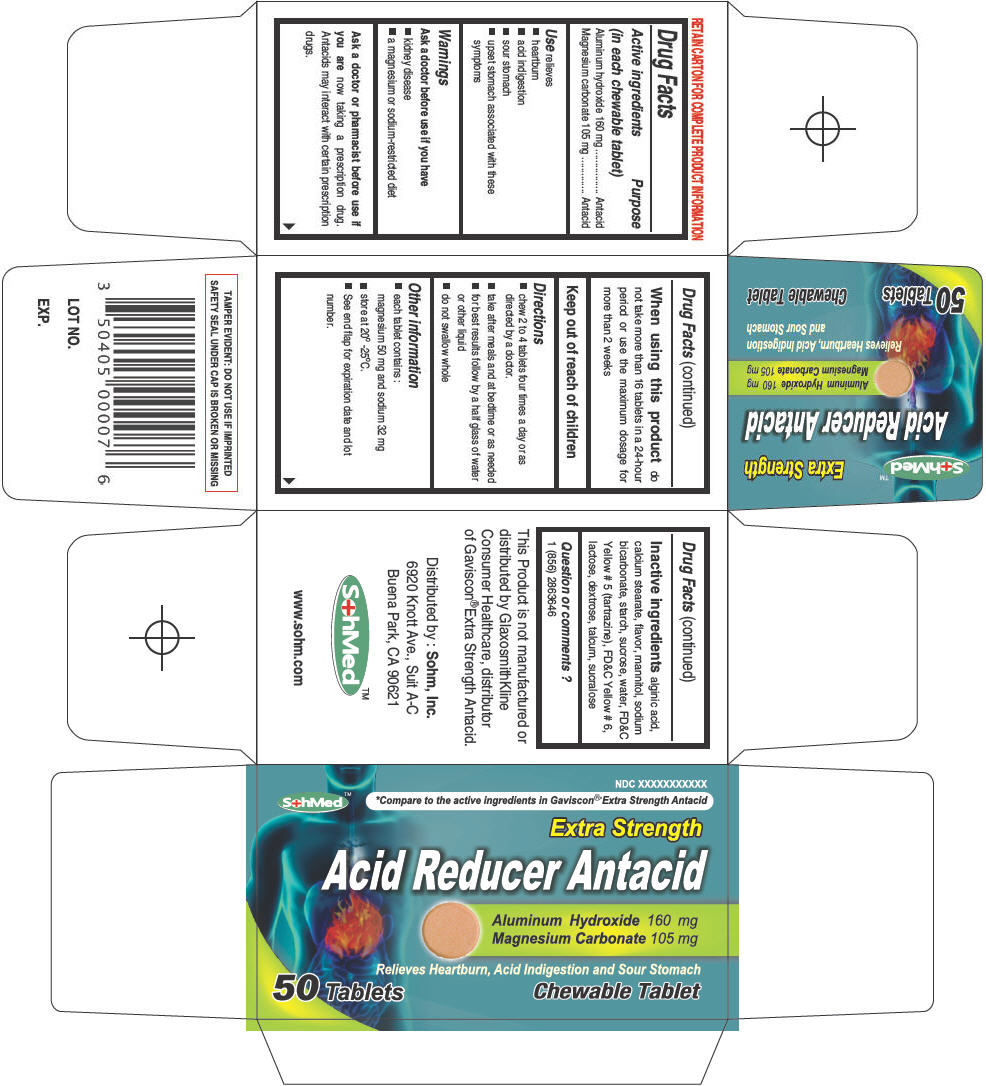
PRINCIPAL DISPLAY PANEL - 50 Tablet Bottle Carton
NDC XXXXXXXXXXX
SohMed™
*Compare to the active ingredients in Gaviscon® Extra Strength Antacid
Extra Strength
Acid Reducer Antacid
Aluminum Hydroxide 160 mg
Magnesium Carbonate 105 mg
Relieves Heartburn, Acid Indigestion and Sour Stomach
50 Tablets
Chewable Tablet