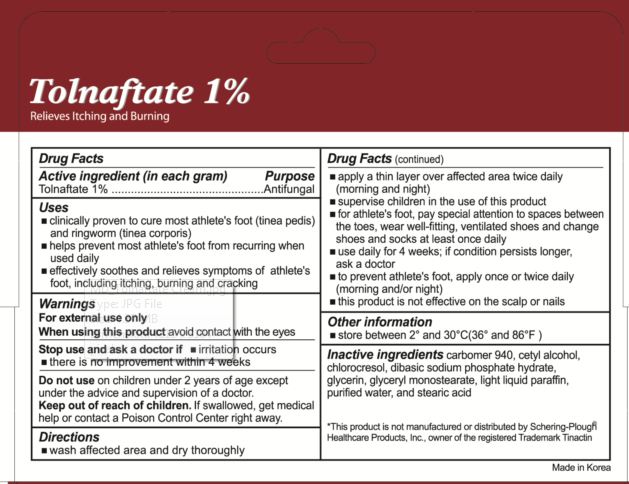
Uses
- clinically proven to cure most athlete's foot (tinea pedis) and ringworm (tinea corporis)
- helps prevent most athlete's foot from recurring when used daily
- effectively soothes and relieves symptoms of athlete's foot, including itching, burning and cracking
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot, pay special attentional to spaces between the toes, wear well-fitting, ventilated shoes and change shoes and socks at least once daily
- use daily for 4 weeks; if condition persists longer ask a doctor
- to prevent athlete's foot, apply once or twice daily (morning and night)
- this product is not effective on the scalp or nails
Other information
store between 2 degrees and 30 degrees Celsius (36 degrees and 86 degrees Fahrenheit)
Inactive ingredients
carbomer 940, cetyl alcohol, chlorocresol, dibasic sodium phosphate hydrate, glycerin, glyceryl monostearate, light liquid paraffin, purified water, and stearic acid
Package label
NDC NO. 53210-1001-0
Tolnafate 1 percent
Cream USP 1 percent Antifungal
Relieves Itching and Burning
Cures and Prevents Athlete's Foot
Prevents and Relieves Itching and Redness
Anti-Itch Cream for Skin Irritations
Compare to Tinactin (R) active ingredient
This product is n ot manufactured or distributed by Schering-Plough Healthcare Products, Inc. owner of the registered Trademark Tinactin
Maximum Strength
Net 1 oz(28.3g)
Exclusively distributed by:
Morales Distributors, Inc
Mayaguez, P.R. 00682
Made in Korea