Use
For Professional and Hospital use. Helps prevent infection. Antiseptic cleansing of face, hands and body without soap and water.
Keep out of reach of children: If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Tear open packet, unfold towelette and use to cleanse desired skin area. Discard towelette appropriately after single use.
Antiseptic Towelette
Genuine First Aid LLC, Clearwater FL 33755
www.GenuineFirstAid.com
1/pouch
GENUINE FIRST AID
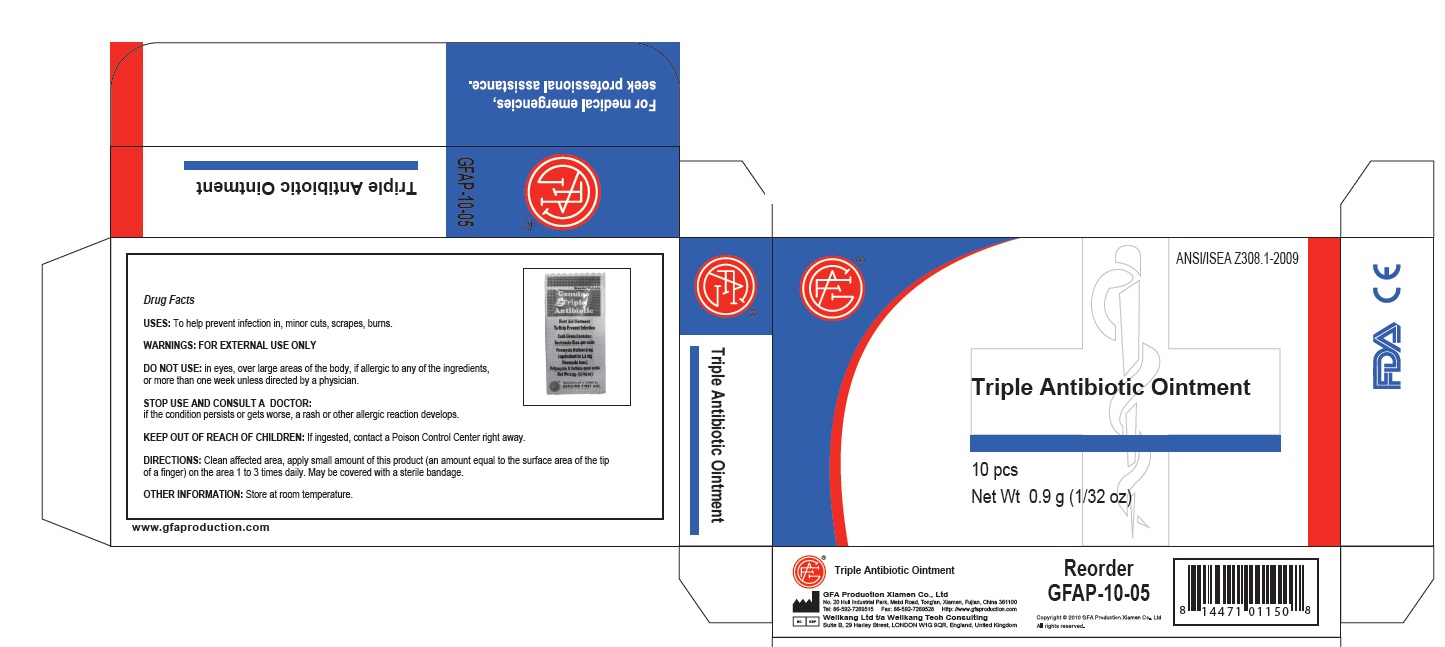
Uses
First aid to help prevent infection and for the temporary relief of pain and itching associated with:
Minor Cuts
Scrapes
Burns
Do not use:
in the eyes or apply over large areas of the body.
longer than 1 week unless directed by a doctor.
in large quantities, particularly over raw surfaces or blistered areas.
Ask a doctor before use if you have deep puncture wounds, animal bites or serious burns.
When using this product, avoid contact with the eyes.
Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days
condition clears up and occurs again within a few days
Directions
Adults and children 2 years of age and older
clean affected area.
apply a small amount of this product on the area 1 to 3 times daily.
may be covered with a sterile bandage
children under 2 years of age: consult a doctor
Other Information:
Store at room temperature (do not freeze).
Taper evident sealed packets.
Do not use packet if opened or torn.
Inactive Ingredients
Aloe vera, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl monostearate, methylparaben, mineral oil, polyethylene glycol, propylene glycol, propylparaben, purified water, stearic acid, irolamine
LOT/EXP: Made in CHINA
20130301
GFA Production Xiamen Co., Ltd
No. 20 Huli Industrial Park, Meixi Road, Tong'an, Xiamen, Fujian, China 361100
Tel: 86-592-7269515 Fax: 86-592-7269528 Http: //www.gfaproduction.com
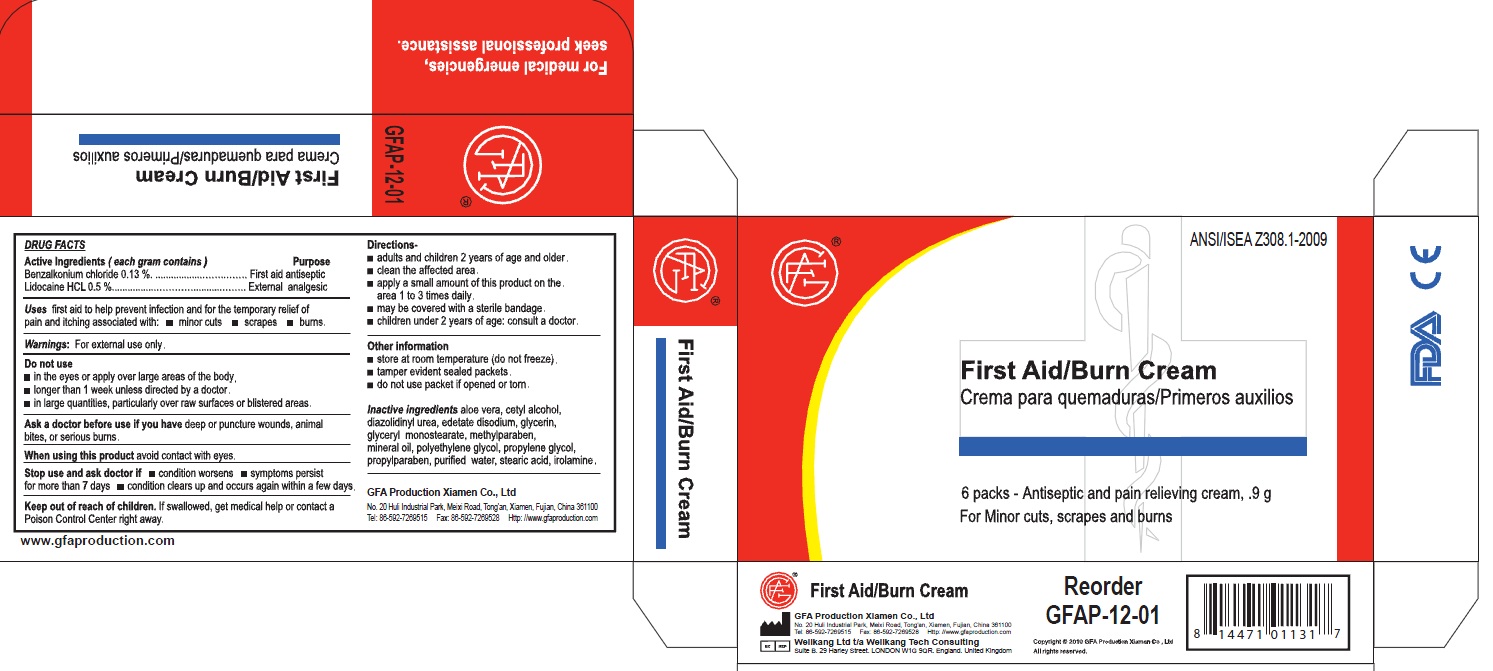
Genuine First Aid Burn Cream
Antiseptic Pain Relief With Aloe
Net Wt 0.9g (1/32 oz)
Manufactured in CHINA for
Genuine First Aid.
Active Ingredients
Active Ingredient: .........Bacitracin Zinc 400 units
Neomycin Sulfate 5mg ( equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate 5000 units
Do not use: in eyes; over large areas of the body;
If allergic to any of the ingredients; for more than one week unless directed by a physician.
Stop use and consult a doctor:
if the condition persists or gets worse; a rash or other allergic reaction develops
Directions
Directions: clean affected area; apply small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily; may be covered with a sterile bandage
Genuine Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram Contains:
Bacitracin Zinc 400 units
Neomycin Sulfate 5 mg
(equivalent to 3.5 mg
Neomycin base)
Polymyxin B Sulfate 5000 units
Net Wt. 0.5g ; (1/64 oz)
Manufactured in CHINA for
GENUINE FIRST AID.
Triple Antibiotic Ointment 10pcs
Net wt. 0.9g (1/32oz)
100
Triple Antibiotic